Annexin 1, glucocorticoids, and the neuroendocrine-immune interface
- PMID: 17192583
- PMCID: PMC1855441
- DOI: 10.1196/annals.1366.002
Annexin 1, glucocorticoids, and the neuroendocrine-immune interface
Abstract
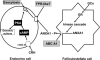
Annexin 1 (ANXA1) was originally identified as a mediator of the anti-inflammatory actions of glucocorticoids (GCs) in the host defense system. Subsequent work confirmed and extended these findings and also showed that the protein fulfills a wider brief and serves as a signaling intermediate in a number of systems. ANXA1 thus contributes to the regulation of processes as diverse as cell migration, cell growth and differentiation, apoptosis, vesicle fusion, lipid metabolism, and cytokine expression. Here we consider the role of ANXA1 in the neuroendocrine system, particularly the hypothalamo-pituitary-adrenocortical (HPA) axis. Evidence is presented that ANXA1 plays a critical role in effecting the negative feedback effects of GCs on the release of corticotrophin (ACTH) and its hypothalamic-releasing hormones and that it is particularly pertinent to the early-onset actions of the steroids that are mediated via a nongenomic mechanism. The paracrine/juxtacrine mode of ANXA1 action is discussed in detail, with particular reference to the significance of the secondary processing of ANXA1, the processes that control the intracellular and transmembrane trafficking of the protein of the molecule and the mechanism of ANXA1 action on its target cells. In addition, the role of ANXA1 in the perinatal programming of the HPA axis is discussed.
Figures





References
-
- Rosengarth A, Luecke H. A calcium-driven conformational switch of the N-terminal and core domains of annexin A1. J. Mol. Biol. 2003;326:1317–1325. - PubMed
-
- Horlick KR, et al. Mouse lipocortin I gene structure and chromosomal assignent: gene duplication and the origins of a gene family. Genomics. 1991;10:365–374. - PubMed
-
- Kovacic RT, et al. Correlation of gene and protein structure of rat and human lipocortin I. Biochemistry. 1991;30:9015–9021. - PubMed
-
- Wallner BP, et al. Cloning and expression of human lipocortin, a phospholipase A2 inhibitor with potential anti-inflammatory activity. Nature. 1986;320:77–81. - PubMed
-
- Smith T, Flower RJ, Buckingham JC. Lipocortins 1, 2 and 5 in the central nervous system and pituitary gland of the rat: selective induction by dexamethasone of lipocortin 1 in the anterior pituitary gland. Mol. Neuropharmacol. 1993;3:45–55.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

