Studying multisite binary and ternary protein interactions by global analysis of isothermal titration calorimetry data in SEDPHAT: application to adaptor protein complexes in cell signaling
- PMID: 17192587
- PMCID: PMC1794685
- DOI: 10.1110/ps.062558507
Studying multisite binary and ternary protein interactions by global analysis of isothermal titration calorimetry data in SEDPHAT: application to adaptor protein complexes in cell signaling
Abstract
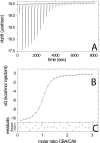
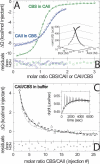
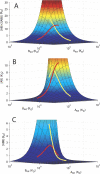
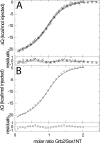
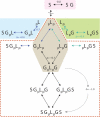
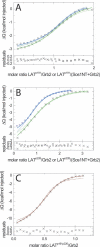
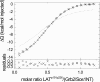
Multisite interactions and the formation of ternary or higher-order protein complexes are ubiquitous features of protein interactions. Cooperativity between different ligands is a hallmark for information transfer, and is frequently critical for the biological function. We describe a new computational platform for the global analysis of isothermal titration calorimetry (ITC) data for the study of binary and ternary multisite interactions, implemented as part of the public domain multimethod analysis software SEDPHAT. The global analysis of titrations performed in different orientations was explored, and the potential for unraveling cooperativity parameters in multisite interactions was assessed in theory and experiment. To demonstrate the practical potential and limitations of global analyses of ITC titrations for the study of cooperative multiprotein interactions, we have examined the interactions of three proteins that are critical for signal transduction after T-cell activation, LAT, Grb2, and Sos1. We have shown previously that multivalent interactions between these three molecules promote the assembly of large multiprotein complexes important for T-cell receptor activation. By global analysis of the heats of binding observed in sets of ITC injections in different orientations, which allowed us to follow the formation of binary and ternary complexes, we observed negative and positive cooperativity that may be important to control the pathway of assembly and disassembly of adaptor protein particles.
Figures

References
-
- Ababou, A. and Ladbury, J.E. 2006. Survey of the year 2004: Literature on applications of isothermal titration calorimetry. J. Mol. Recognit. 19: 79–89. - PubMed
-
- Arnaud, A. and Bouteiller, L. 2004. Isothermal titration calorimetry of supramolecular polymers. Langmuir 20: 6858–6863. - PubMed
-
- Bains, G. and Freire, E. 1991. Calorimetric determination of cooperative interactions in high affinity binding processes. Anal. Biochem. 192: 203–206. - PubMed
-
- Baker, B.M. and Murphy, K.P. 1997. Dissecting the energetics of a protein-protein interaction: The binding of ovomucoid third domain to elastase. J. Mol. Biol. 268: 557–569. - PubMed
-
- Baker, B.M. and Murphy, K.P. 1998. Prediction of binding energetics from structure using empirical parameterization. Methods Enzymol. 295: 294–315. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

