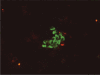
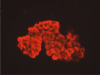
Diagnostic Utility of the ImmunoCyt/uCyt+ Test in Bladder Cancer
- PMID: 17192798
- PMCID: PMC1751037
Diagnostic Utility of the ImmunoCyt/uCyt+ Test in Bladder Cancer
Abstract
Bladder cancer is a common malignancy in the United States. Although urine cytology is a useful adjunct in both diagnosis and follow-up and is highly sensitive for detecting high-grade tumors, it is limited by decreased sensitivity in detecting low-grade tumors, which constitute the majority of new diagnoses. Additional screening tests with high sensitivity and specificity for urothelial tumors of all grades are indicated to help improve the diagnostic ability of urine cytology as well as to reduce the need for frequent cystoscopies, especially in those with low-risk disease. Several assays have been developed, with the ImmunoCyt/uCyt+ test (DiagnoCure, Inc., Québec, Canada) being especially promising. Recent studies on the applicability and efficacy of ImmunoCyt/uCyt+ testing are reviewed, as are its sensitivity, specificity, and predictive value in the follow-up and screening of urothelial malignancies.
Figures
Similar articles
-
ImmunoCyt/uCyt+ improves the sensitivity of urine cytology in patients followed for urothelial carcinoma.Mod Pathol. 2005 Jan;18(1):83-9. doi: 10.1038/modpathol.3800262. Mod Pathol. 2005. PMID: 15389253
-
uCyt+/ImmunoCyt and cytology in the detection of urothelial carcinoma: an update on 7422 analyses.Cancer Cytopathol. 2013 Jul;121(7):392-7. doi: 10.1002/cncy.21287. Epub 2013 Mar 12. Cancer Cytopathol. 2013. PMID: 23495066
-
The value of the ImmunoCyt/uCyt+ test in the detection and follow-up of carcinoma in situ of the urinary bladder.Anticancer Res. 2005 Sep-Oct;25(5):3641-4. Anticancer Res. 2005. PMID: 16101194
-
Replacing cystoscopy by urine markers in the follow-up of patients with low-risk non-muscle-invasive bladder cancer?-An International Bladder Cancer Network project.Urol Oncol. 2016 Oct;34(10):452-9. doi: 10.1016/j.urolonc.2016.06.001. Epub 2016 Jul 2. Urol Oncol. 2016. PMID: 27381893 Review.
-
Current bladder tumor tests: does their projected utility fulfill clinical necessity?J Urol. 2001 Apr;165(4):1067-77. J Urol. 2001. PMID: 11257640 Review.
Cited by
-
Urinary Biomarkers in Bladder Cancer: Where Do We Stand and Potential Role of Extracellular Vesicles.Cancers (Basel). 2020 May 29;12(6):1400. doi: 10.3390/cancers12061400. Cancers (Basel). 2020. PMID: 32485907 Free PMC article. Review.
-
Biomarkers for Precision Urothelial Carcinoma Diagnosis: Current Approaches and the Application of Single-Cell Technologies.Cancers (Basel). 2021 Jan 12;13(2):260. doi: 10.3390/cancers13020260. Cancers (Basel). 2021. PMID: 33445605 Free PMC article. Review.
-
Individual risk assessment in bladder cancer patients based on a multi-marker panel.J Cancer Res Clin Oncol. 2013 Jan;139(1):49-56. doi: 10.1007/s00432-012-1297-9. Epub 2012 Aug 15. J Cancer Res Clin Oncol. 2013. PMID: 22893018 Free PMC article.
-
Urine Biopsy as Dynamic Biomarker to Enhance Clinical Staging of Bladder Cancer in Radical Cystectomy Candidates.JCO Precis Oncol. 2024 Jun;8:e2300362. doi: 10.1200/PO.23.00362. JCO Precis Oncol. 2024. PMID: 38865671 Free PMC article.
-
Proteomic research and diagnosis in bladder cancer: state of the art review.Int Braz J Urol. 2021 May-Jun;47(3):503-514. doi: 10.1590/S1677-5538.IBJU.2021.99.02. Int Braz J Urol. 2021. PMID: 32459456 Free PMC article. Review.
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. - PubMed
-
- Smith JA , Jr, Labasky RF, Cockett AT, et al. Bladder cancer clinical guidelines panel summary report on the management of nonmuscle invasive bladder cancer (stages Ta, T1 and TIS). The American Urological Association. J Urol. 1999;162:1697–1701. - PubMed
-
- Lotan Y, Roehrborn CG. Cost-effectiveness of a modified care protocol substituting bladder tumor markers for cystoscopy for the followup of patients with transitional cell carcinoma of the bladder: a decision analytical approach. J Urol. 2002;167:75–79. - PubMed
-
- Bastacky S, Ibrahim S, Wilczynski SP, et al. The accuracy of urinary cytology in daily practice. Cancer. 1999;87:118–128. - PubMed
-
- Fradet Y, Lockhard C. Performance characteristics of a new monoclonal antibody test for bladder cancer: ImmunoCyt trade mark. Can J Urol. 1997;4:400–405. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources