Kinetic analysis of hyaluronidase activity using a bioactive MRI contrast agent
- PMID: 17193686
- PMCID: PMC4035508
- DOI: 10.1002/cmmi.96
Kinetic analysis of hyaluronidase activity using a bioactive MRI contrast agent
Abstract
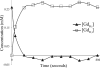
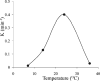
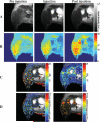
One of the attractions of molecular imaging using 'smart' bioactive contrast agents is the ability to provide non-invasive data on the spatial and temporal changes in the distribution and expression patterns of specific enzymes. The tools developed for that aim could potentially also be developed for functional imaging of enzyme activity itself, through quantitative analysis of the rapid dynamics of enzymatic conversion of these contrast agents. High molecular weight hyaluronan, the natural substrate of hyaluronidase, is a major antiangiogenic constituent of the extracellular matrix. Degradation by hyaluronidase yields low molecular weight fragments, which are proangiogenic. A novel contrast material, HA-GdDTPA-beads, was designed to provide a substrate analog of hyaluronidase in which relaxivity changes are induced by enzymatic degradation. We show here a first-order kinetic analysis of the time-dependent increase in R(2) as a result of hyaluronidase activity. The changes in R(2) and the measured relaxivity of intact HA-GdDTPA-beads (r(2B)) and HA-GdDTPA fragments (r(2D)) were utilized for derivation of the temporal drop in concentration of GdDTPA in HA-GdDTPA-beads as the consequence of the release of HA-GdDTPA fragments. The rate of dissociation of HA-GdDTPA from the beads showed typical bell-shaped temperature dependence between 7 and 36 degrees C with peak activity at 25 degrees C. The tools developed here for quantitative dynamic analysis of hyaluronidase activity by MRI would allow the use of activation of HA-GdDTPA-beads for the determination of the role of hyaluronidase in altering the angiogenic microenvironment of tumor micro metastases.
Copyright 2006 John Wiley & Sons, Ltd.
Figures

Similar articles
-
Emerging roles for hyaluronidase in cancer metastasis and therapy.Adv Cancer Res. 2014;123:1-34. doi: 10.1016/B978-0-12-800092-2.00001-0. Adv Cancer Res. 2014. PMID: 25081524 Free PMC article. Review.
-
Magnetic resonance imaging visualization of hyaluronidase in ovarian carcinoma.Cancer Res. 2005 Nov 15;65(22):10316-23. doi: 10.1158/0008-5472.CAN-04-3947. Cancer Res. 2005. PMID: 16288020
-
Gadolinium-conjugated CB86: a novel TSPO-targeting MRI contrast agent for imaging of rheumatoid arthritis.J Drug Target. 2020 Apr;28(4):398-407. doi: 10.1080/1061186X.2019.1669040. Epub 2019 Nov 12. J Drug Target. 2020. PMID: 31530199
-
Peptide-conjugated polyamidoamine dendrimer as a nanoscale tumor-targeted T1 magnetic resonance imaging contrast agent.Biomaterials. 2011 Apr;32(11):2989-98. doi: 10.1016/j.biomaterials.2011.01.005. Epub 2011 Jan 28. Biomaterials. 2011. PMID: 21277017
-
The magic glue hyaluronan and its eraser hyaluronidase: a biological overview.Life Sci. 2007 May 1;80(21):1921-43. doi: 10.1016/j.lfs.2007.02.037. Epub 2007 Mar 6. Life Sci. 2007. PMID: 17408700 Review.
Cited by
-
Emerging roles for hyaluronidase in cancer metastasis and therapy.Adv Cancer Res. 2014;123:1-34. doi: 10.1016/B978-0-12-800092-2.00001-0. Adv Cancer Res. 2014. PMID: 25081524 Free PMC article. Review.
-
Detecting enzyme activities with exogenous MRI contrast agents.Chemistry. 2014 Aug 4;20(32):9840-50. doi: 10.1002/chem.201402474. Epub 2014 Jul 2. Chemistry. 2014. PMID: 24990812 Free PMC article. Review.
-
Hyaluronidase activity of human Hyal1 requires active site acidic and tyrosine residues.J Biol Chem. 2009 Apr 3;284(14):9433-42. doi: 10.1074/jbc.M900210200. Epub 2009 Feb 6. J Biol Chem. 2009. PMID: 19201751 Free PMC article.
-
Molecular imaging and targeted therapies.Biochem Pharmacol. 2010 Sep 1;80(5):731-8. doi: 10.1016/j.bcp.2010.04.011. Epub 2010 Apr 21. Biochem Pharmacol. 2010. PMID: 20399197 Free PMC article. Review.
-
MRI Contrast Agents in Glycobiology.Molecules. 2022 Nov 28;27(23):8297. doi: 10.3390/molecules27238297. Molecules. 2022. PMID: 36500389 Free PMC article. Review.
References
-
- Schiffenbauer YS, Meir G, Maoz M, Even-Ram SC, Bar-Shavit R, Neeman M. Gonadotropin stimulation of MLS human epithelial ovarian carcinoma cells augments cell adhesion mediated by CD44 and by alpha(v)-integrin. Gynecol. Oncol. 2002;84:296–302. - PubMed
-
- Cohen M, Joester D, Geiger B, Addadi L. Spatial and temporal sequence of events in cell adhesion: from molecular recognition to focal adhesion assembly. ChemBiochem. 2004;5:1393–1399. - PubMed
-
- Tempel C, Gilead A, Neeman M. Hyaluronic acid as an anti-angiogenic shield in the preovulatory rat follicle. Biol. Reprod. 2000;63:134–140. - PubMed
-
- West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228:1324–1326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
