Palladin mutation causes familial pancreatic cancer and suggests a new cancer mechanism
- PMID: 17194196
- PMCID: PMC1751121
- DOI: 10.1371/journal.pmed.0030516
Palladin mutation causes familial pancreatic cancer and suggests a new cancer mechanism
Abstract
Background: Pancreatic cancer is a deadly disease. Discovery of the mutated genes that cause the inherited form(s) of the disease may shed light on the mechanism(s) of oncogenesis. Previously we isolated a susceptibility locus for familial pancreatic cancer to chromosome location 4q32-34. In this study, our goal was to discover the identity of the familial pancreatic cancer gene on 4q32 and determine the function of that gene.
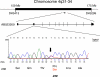
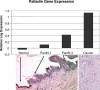
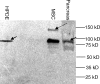
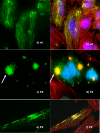
Methods and findings: A customized microarray of the candidate chromosomal region affecting pancreatic cancer susceptibility revealed the greatest expression change in palladin (PALLD), a gene that encodes a component of the cytoskeleton that controls cell shape and motility. A mutation causing a proline (hydrophobic) to serine (hydrophilic) amino acid change (P239S) in a highly conserved region tracked with all affected family members and was absent in the non-affected members. The mutational change is not a known single nucleotide polymorphism. Palladin RNA, measured by quantitative RT-PCR, was overexpressed in the tissues from precancerous dysplasia and pancreatic adenocarcinoma in both familial and sporadic disease. Transfection of wild-type and P239S mutant palladin gene constructs into HeLa cells revealed a clear phenotypic effect: cells expressing P239S palladin exhibited cytoskeletal changes, abnormal actin bundle assembly, and an increased ability to migrate.
Conclusions: These observations suggest that the presence of an abnormal palladin gene in familial pancreatic cancer and the overexpression of palladin protein in sporadic pancreatic cancer cause cytoskeletal changes in pancreatic cancer and may be responsible for or contribute to the tumor's strong invasive and migratory abilities.
Conflict of interest statement
Figures





Comment in
-
Palladin mutation causes familial pancreatic cancer: absence in European families.PLoS Med. 2007 Apr;4(4):e164. doi: 10.1371/journal.pmed.0040164. PLoS Med. 2007. PMID: 17455999 Free PMC article. No abstract available.
Similar articles
-
The role of palladin in actin organization and cell motility.Eur J Cell Biol. 2008 Sep;87(8-9):517-25. doi: 10.1016/j.ejcb.2008.01.010. Epub 2008 Mar 14. Eur J Cell Biol. 2008. PMID: 18342394 Free PMC article. Review.
-
[Familial pancreatic cancer and the palladin gene: a new look at cancer mechanisms].Med Sci (Paris). 2007 Mar;23(3):232-4. doi: 10.1051/medsci/2007233232. Med Sci (Paris). 2007. PMID: 17349274 French. No abstract available.
-
The P239S palladin variant does not account for a significant fraction of hereditary or early onset pancreas cancer.Hum Genet. 2007 Jun;121(5):635-7. doi: 10.1007/s00439-007-0361-z. Epub 2007 Apr 6. Hum Genet. 2007. PMID: 17415588
-
Palladin is overexpressed in the non-neoplastic stroma of infiltrating ductal adenocarcinomas of the pancreas, but is only rarely overexpressed in neoplastic cells.Cancer Biol Ther. 2007 Mar;6(3):324-8. doi: 10.4161/cbt.6.3.3904. Epub 2007 Mar 24. Cancer Biol Ther. 2007. PMID: 17404500 Free PMC article.
-
Molecular genetic alterations in ductal pancreatic adenocarcinomas.Med Clin North Am. 2000 May;84(3):691-5, xi. doi: 10.1016/s0025-7125(05)70251-0. Med Clin North Am. 2000. PMID: 10872425 Review.
Cited by
-
Genome-wide Association Study Identifies Novel Risk Loci for Apical Periodontitis.J Endod. 2023 Oct;49(10):1276-1288. doi: 10.1016/j.joen.2023.07.018. Epub 2023 Jul 25. J Endod. 2023. PMID: 37499862 Free PMC article.
-
Risk factors and therapeutic targets in pancreatic cancer.Front Oncol. 2013 Nov 18;3:282. doi: 10.3389/fonc.2013.00282. Front Oncol. 2013. PMID: 24303367 Free PMC article. Review.
-
Palladin regulation of the actin structures needed for cancer invasion.Cell Adh Migr. 2014;8(1):29-35. doi: 10.4161/cam.28024. Epub 2013 Jan 1. Cell Adh Migr. 2014. PMID: 24525547 Free PMC article. Review.
-
The role of palladin in actin organization and cell motility.Eur J Cell Biol. 2008 Sep;87(8-9):517-25. doi: 10.1016/j.ejcb.2008.01.010. Epub 2008 Mar 14. Eur J Cell Biol. 2008. PMID: 18342394 Free PMC article. Review.
-
Precursors to pancreatic cancer.Gastroenterol Clin North Am. 2007 Dec;36(4):831-49, vi. doi: 10.1016/j.gtc.2007.08.012. Gastroenterol Clin North Am. 2007. PMID: 17996793 Free PMC article. Review.
References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. - PubMed
-
- Shaib YH, Davila JA, El Serag HB. The epidemiology of pancreatic cancer in the United States: Changes below the surface. Aliment Pharmacol Ther. 2006;24:87–94. - PubMed
-
- Hruban RH, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJ, et al. Familial pancreatic cancer. Ann Oncol. 1999;10((Suppl 4)):69–73. - PubMed
-
- Rulyak SJ, Brentnall TA. Inherited pancreatic cancer: Improvements in our understanding of genetics and screening. Int J Biochem Cell Biol. 2004;36:1386–1392. - PubMed
-
- Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634–2638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

