An HIV feedback resistor: auto-regulatory circuit deactivator and noise buffer
- PMID: 17194214
- PMCID: PMC1717016
- DOI: 10.1371/journal.pbio.0050009
An HIV feedback resistor: auto-regulatory circuit deactivator and noise buffer
Abstract
Animal viruses (e.g., lentiviruses and herpesviruses) use transcriptional positive feedback (i.e., transactivation) to regulate their gene expression. But positive-feedback circuits are inherently unstable when turned off, which presents a particular dilemma for latent viruses that lack transcriptional repressor motifs. Here we show that a dissipative feedback resistor, composed of enzymatic interconversion of the transactivator, converts transactivation circuits into excitable systems that generate transient pulses of expression, which decay to zero. We use HIV-1 as a model system and analyze single-cell expression kinetics to explore whether the HIV-1 transactivator of transcription (Tat) uses a resistor to shut off transactivation. The Tat feedback circuit was found to lack bi-stability and Tat self-cooperativity but exhibited a pulse of activity upon transactivation, all in agreement with the feedback resistor model. Guided by a mathematical model, biochemical and genetic perturbation of the suspected Tat feedback resistor altered the circuit's stability and reduced susceptibility to molecular noise, in agreement with model predictions. We propose that the feedback resistor is a necessary, but possibly not sufficient, condition for turning off noisy transactivation circuits lacking a repressor motif (e.g., HIV-1 Tat). Feedback resistors may be a paradigm for examining other auto-regulatory circuits and may inform upon how viral latency is established, maintained, and broken.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures


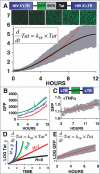
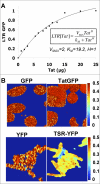
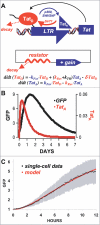
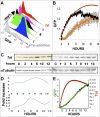
Comment in
-
A simple feedback resistor switch keeps latent HIV from awakening.PLoS Biol. 2007 Jan;5(1):e25. doi: 10.1371/journal.pbio.0050025. Epub 2006 Dec 26. PLoS Biol. 2007. PMID: 20076644 Free PMC article. No abstract available.
Similar articles
-
Distinct promoter activation mechanisms modulate noise-driven HIV gene expression.Sci Rep. 2015 Dec 15;5:17661. doi: 10.1038/srep17661. Sci Rep. 2015. PMID: 26666681 Free PMC article.
-
Transient-mediated fate determination in a transcriptional circuit of HIV.Nat Genet. 2008 Apr;40(4):466-70. doi: 10.1038/ng.116. Epub 2008 Mar 16. Nat Genet. 2008. PMID: 18344999
-
Nonlatching positive feedback enables robust bimodality by decoupling expression noise from the mean.PLoS Biol. 2017 Oct 18;15(10):e2000841. doi: 10.1371/journal.pbio.2000841. eCollection 2017 Oct. PLoS Biol. 2017. PMID: 29045398 Free PMC article.
-
Fate-Regulating Circuits in Viruses: From Discovery to New Therapy Targets.Annu Rev Virol. 2017 Sep 29;4(1):469-490. doi: 10.1146/annurev-virology-110615-035606. Epub 2017 Aug 11. Annu Rev Virol. 2017. PMID: 28800289 Free PMC article. Review.
-
Regulation of expression of human immunodeficiency virus.New Biol. 1990 Jan;2(1):20-31. New Biol. 1990. PMID: 2078551 Review.
Cited by
-
Transcriptional and posttranscriptional regulation of HIV-1 gene expression.Cold Spring Harb Perspect Med. 2012 Feb;2(2):a006916. doi: 10.1101/cshperspect.a006916. Cold Spring Harb Perspect Med. 2012. PMID: 22355797 Free PMC article. Review.
-
Loss of the Brm-type SWI/SNF chromatin remodeling complex is a strong barrier to the Tat-independent transcriptional elongation of human immunodeficiency virus type 1 transcripts.J Virol. 2009 Nov;83(22):11569-80. doi: 10.1128/JVI.00742-09. Epub 2009 Sep 2. J Virol. 2009. PMID: 19726504 Free PMC article.
-
Temperature control of fimbriation circuit switch in uropathogenic Escherichia coli: quantitative analysis via automated model abstraction.PLoS Comput Biol. 2010 Mar 26;6(3):e1000723. doi: 10.1371/journal.pcbi.1000723. PLoS Comput Biol. 2010. PMID: 20361050 Free PMC article.
-
Transcriptional Stochasticity as a Key Aspect of HIV-1 Latency.Viruses. 2023 Sep 21;15(9):1969. doi: 10.3390/v15091969. Viruses. 2023. PMID: 37766375 Free PMC article. Review.
-
Distinct promoter activation mechanisms modulate noise-driven HIV gene expression.Sci Rep. 2015 Dec 15;5:17661. doi: 10.1038/srep17661. Sci Rep. 2015. PMID: 26666681 Free PMC article.
References
-
- Ptashne M. A genetic switch: Phage lambda revisited. Cold Spring Harbor (NewYork): Cold Spring Harbor Laboratory Press; 2004. 154
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

