DARPP-32 is a robust integrator of dopamine and glutamate signals
- PMID: 17194217
- PMCID: PMC1761654
- DOI: 10.1371/journal.pcbi.0020176
DARPP-32 is a robust integrator of dopamine and glutamate signals
Abstract
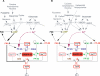
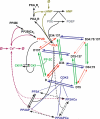
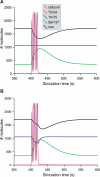
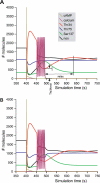
Integration of neurotransmitter and neuromodulator signals in the striatum plays a central role in the functions and dysfunctions of the basal ganglia. DARPP-32 is a key actor of this integration in the GABAergic medium-size spiny neurons, in particular in response to dopamine and glutamate. When phosphorylated by cAMP-dependent protein kinase (PKA), DARPP-32 inhibits protein phosphatase-1 (PP1), whereas when phosphorylated by cyclin-dependent kinase 5 (CDK5) it inhibits PKA. DARPP-32 is also regulated by casein kinases and by several protein phosphatases. These complex and intricate regulations make simple predictions of DARPP-32 dynamic behaviour virtually impossible. We used detailed quantitative modelling of the regulation of DARPP-32 phosphorylation to improve our understanding of its function. The models included all the combinations of the three best-characterized phosphorylation sites of DARPP-32, their regulation by kinases and phosphatases, and the regulation of those enzymes by cAMP and Ca(2+) signals. Dynamic simulations allowed us to observe the temporal relationships between cAMP and Ca(2+) signals. We confirmed that the proposed regulation of protein phosphatase-2A (PP2A) by calcium can account for the observed decrease of Threonine 75 phosphorylation upon glutamate receptor activation. DARPP-32 is not simply a switch between PP1-inhibiting and PKA-inhibiting states. Sensitivity analysis showed that CDK5 activity is a major regulator of the response, as previously suggested. Conversely, the strength of the regulation of PP2A by PKA or by calcium had little effect on the PP1-inhibiting function of DARPP-32 in these conditions. The simulations showed that DARPP-32 is not only a robust signal integrator, but that its response also depends on the delay between cAMP and calcium signals affecting the response to the latter. This integration did not depend on the concentration of DARPP-32, while the absolute effect on PP1 varied linearly. In silico mutants showed that Ser137 phosphorylation affects the influence of the delay between dopamine and glutamate, and that constitutive phosphorylation in Ser137 transforms DARPP-32 in a quasi-irreversible switch. This work is a first attempt to better understand the complex interactions between cAMP and Ca(2+) regulation of DARPP-32. Progressive inclusion of additional components should lead to a realistic model of signalling networks underlying the function of striatal neurons.
Conflict of interest statement
Figures


Similar articles
-
Glutamate Counteracts Dopamine/PKA Signaling via Dephosphorylation of DARPP-32 Ser-97 and Alteration of Its Cytonuclear Distribution.J Biol Chem. 2017 Jan 27;292(4):1462-1476. doi: 10.1074/jbc.M116.752402. Epub 2016 Dec 20. J Biol Chem. 2017. PMID: 27998980 Free PMC article.
-
DARPP-32 40 years later.Adv Pharmacol. 2021;90:67-87. doi: 10.1016/bs.apha.2020.09.004. Epub 2020 Dec 2. Adv Pharmacol. 2021. PMID: 33706939 Review.
-
Transient calcium and dopamine increase PKA activity and DARPP-32 phosphorylation.PLoS Comput Biol. 2006 Sep 8;2(9):e119. doi: 10.1371/journal.pcbi.0020119. PLoS Comput Biol. 2006. PMID: 16965177 Free PMC article.
-
Muscarinic acetylcholine M4 receptors play a critical role in oxotremorine-induced DARPP-32 phosphorylation at threonine 75 in isolated medium spiny neurons.Neuropharmacology. 2017 May 1;117:376-386. doi: 10.1016/j.neuropharm.2017.02.026. Epub 2017 Mar 1. Neuropharmacology. 2017. PMID: 28257887
-
DARPP-32: an integrator of neurotransmission.Annu Rev Pharmacol Toxicol. 2004;44:269-96. doi: 10.1146/annurev.pharmtox.44.101802.121415. Annu Rev Pharmacol Toxicol. 2004. PMID: 14744247 Review.
Cited by
-
Exploring the Role of DARPP-32 in Addiction: A Review of the Current Limitations of Addiction Treatment Pathways and the Role of DARPP-32 to Improve Them.NeuroSci. 2022 Aug 25;3(3):494-509. doi: 10.3390/neurosci3030035. eCollection 2022 Sep. NeuroSci. 2022. PMID: 39483434 Free PMC article. Review.
-
STEPS: efficient simulation of stochastic reaction-diffusion models in realistic morphologies.BMC Syst Biol. 2012 May 10;6:36. doi: 10.1186/1752-0509-6-36. BMC Syst Biol. 2012. PMID: 22574658 Free PMC article.
-
Dendritic geometry shapes neuronal cAMP signalling to the nucleus.Nat Commun. 2015 Feb 18;6:6319. doi: 10.1038/ncomms7319. Nat Commun. 2015. PMID: 25692798 Free PMC article.
-
Neuronal Glutamate Transporters Control Dopaminergic Signaling and Compulsive Behaviors.J Neurosci. 2018 Jan 24;38(4):937-961. doi: 10.1523/JNEUROSCI.1906-17.2017. Epub 2017 Dec 11. J Neurosci. 2018. PMID: 29229708 Free PMC article.
-
Microfluidics delivery of DARPP-32 into HeLa cells maintains viability for in-cell NMR spectroscopy.Commun Biol. 2022 May 12;5(1):451. doi: 10.1038/s42003-022-03412-x. Commun Biol. 2022. PMID: 35551287 Free PMC article.
References
-
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20:91–127. - PubMed
-
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. - PubMed
-
- Spencer HJ. Antagonism of cortical excitation of striatal neurons by glutamic acid diethyl ester: Evidence for glutamic acid as an excitatory transmitter in the rat striatum. Brain Res. 1976;102:91–101. - PubMed
-
- Girault JA, Spampinato U, Savaki HE, Glowinski J, Besson MJ. In vivo release of [3H]gamma-aminobutyric acid in the rat neostriatum—I. Characterization and topographical heterogeneity of the effects of dopaminergic and cholinergic agents. Neuroscience. 1986;19:1101–1108. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

