Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis
- PMID: 17194222
- PMCID: PMC1713256
- DOI: 10.1371/journal.pgen.0020216
Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis
Abstract
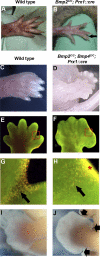
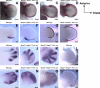
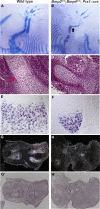
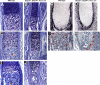
Bone morphogenetic protein (BMP) family members, including BMP2, BMP4, and BMP7, are expressed throughout limb development. BMPs have been implicated in early limb patterning as well as in the process of skeletogenesis. However, due to complications associated with early embryonic lethality, particularly for Bmp2 and Bmp4, and with functional redundancy among BMP molecules, it has been difficult to decipher the specific roles of these BMP molecules during different stages of limb development. To circumvent these issues, we have constructed a series of mouse strains lacking one or more of these BMPs, using conditional alleles in the case of Bmp2 and Bmp4 to remove them specifically from the limb bud mesenchyme. Contrary to earlier suggestions, our results indicate that BMPs neither act as secondary signals downstream of Sonic Hedghog (SHH) in patterning the anteroposterior axis nor as signals from the interdigital mesenchyme in specifying digit identity. We do find that a threshold level of BMP signaling is required for the onset of chondrogenesis, and hence some chondrogenic condensations fail to form in limbs deficient in both BMP2 and BMP4. However, in the condensations that do form, subsequent chondrogenic differentiation proceeds normally even in the absence of BMP2 and BMP7 or BMP2 and BMP4. In contrast, we find that the loss of both BMP2 and BMP4 results in a severe impairment of osteogenesis.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures



References
-
- Urist MR. Bone: Formation by autoinduction. Science. 1965;150:893–899. - PubMed
-
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, et al. Novel regulators of bone formation: Molecular clones and activities. Science. 1988;242:1528–1534. - PubMed
-
- Hogan BL. Bone morphogenetic proteins: Multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. - PubMed
-
- Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

