Lipid rafts play an important role in the early stage of severe acute respiratory syndrome-coronavirus life cycle
- PMID: 17194611
- PMCID: PMC7110773
- DOI: 10.1016/j.micinf.2006.10.015
Lipid rafts play an important role in the early stage of severe acute respiratory syndrome-coronavirus life cycle
Abstract
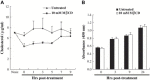
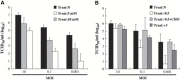
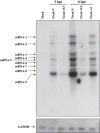
Lipid rafts are involved in the life cycle of many viruses. In this study, we showed that lipid rafts also play an important role in the life cycle of severe acute respiratory syndrome (SARS)-coronavirus (CoV). Cholesterol depletion by pretreatment of Vero E6 cells with methyl-beta-cyclodextrin (MbetaCD) inhibited the production of SARS-CoV particles released from the infected cells. This inhibition was prevented by addition of cholesterol to the culture medium, indicating that the reduction of virus particle release was caused by the loss of cholesterol in the cell membrane. In contrast, cholesterol depletion at the post-entry stage (3h post-infection) caused only a limited effect on virus particle release. Northern blot analysis revealed that the levels of viral mRNAs were significantly affected by pretreatment with MbetaCD, but not by treatment at 3h post-infection. Interestingly, no apparent evidence for colocalization of angiotensin converting enzyme 2 with lipid rafts in the membrane of Vero E6 cells was obtained. These results suggest that lipid rafts could contribute to SARS-CoV infection in the early replication process in Vero E6 cells.
Figures

Similar articles
-
Importance of cholesterol-rich membrane microdomains in the interaction of the S protein of SARS-coronavirus with the cellular receptor angiotensin-converting enzyme 2.Virology. 2008 Nov 25;381(2):215-21. doi: 10.1016/j.virol.2008.08.026. Epub 2008 Sep 23. Virology. 2008. PMID: 18814896 Free PMC article.
-
Lipid rafts are involved in SARS-CoV entry into Vero E6 cells.Biochem Biophys Res Commun. 2008 May 2;369(2):344-9. doi: 10.1016/j.bbrc.2008.02.023. Epub 2008 Feb 13. Biochem Biophys Res Commun. 2008. PMID: 18279660 Free PMC article.
-
SARS-CoV-2 entry and fusion are independent of ACE2 localization to lipid rafts.J Virol. 2025 Jan 31;99(1):e0182324. doi: 10.1128/jvi.01823-24. Epub 2024 Nov 21. J Virol. 2025. PMID: 39570043 Free PMC article.
-
SARS-CoV replication and pathogenesis in an in vitro model of the human conducting airway epithelium.Virus Res. 2008 Apr;133(1):33-44. doi: 10.1016/j.virusres.2007.03.013. Epub 2007 Apr 23. Virus Res. 2008. PMID: 17451829 Free PMC article. Review.
-
Cholesterol-Rich Lipid Rafts as Platforms for SARS-CoV-2 Entry.Front Immunol. 2021 Dec 16;12:796855. doi: 10.3389/fimmu.2021.796855. eCollection 2021. Front Immunol. 2021. PMID: 34975904 Free PMC article. Review.
Cited by
-
Coronavirus Interplay With Lipid Rafts and Autophagy Unveils Promising Therapeutic Targets.Front Microbiol. 2020 Aug 11;11:1821. doi: 10.3389/fmicb.2020.01821. eCollection 2020. Front Microbiol. 2020. PMID: 32849425 Free PMC article. Review.
-
Cyclic Oligosaccharides as Active Drugs, an Updated Review.Pharmaceuticals (Basel). 2020 Sep 29;13(10):281. doi: 10.3390/ph13100281. Pharmaceuticals (Basel). 2020. PMID: 33003610 Free PMC article. Review.
-
A spatial multi-scale fluorescence microscopy toolbox discloses entry checkpoints of SARS-CoV-2 variants in Vero E6 cells.Comput Struct Biotechnol J. 2021;19:6140-6156. doi: 10.1016/j.csbj.2021.10.038. Epub 2021 Nov 2. Comput Struct Biotechnol J. 2021. PMID: 34745450 Free PMC article.
-
PEDV enters cells through clathrin-, caveolae-, and lipid raft-mediated endocytosis and traffics via the endo-/lysosome pathway.Vet Res. 2020 Feb 10;51(1):10. doi: 10.1186/s13567-020-0739-7. Vet Res. 2020. PMID: 32041637 Free PMC article.
-
The potential role of scavenger receptor B type I (SR-BI) in SARS-CoV-2 infection.Immun Inflamm Dis. 2023 Apr;11(4):e786. doi: 10.1002/iid3.786. Immun Inflamm Dis. 2023. PMID: 37102664 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

