Phosphorylation of MNAR promotes estrogen activation of phosphatidylinositol 3-kinase
- PMID: 17194752
- PMCID: PMC1820473
- DOI: 10.1128/MCB.01732-06
Phosphorylation of MNAR promotes estrogen activation of phosphatidylinositol 3-kinase
Retraction in
-
Phosphorylation of MNAR promotes estrogen activation of phosphatidylinositol 3-kinase. Retraction.Mol Cell Biol. 2010 Mar;30(6):1568. doi: 10.1128/MCB.00090-10. Mol Cell Biol. 2010. PMID: 20194629 Free PMC article. No abstract available.
Abstract
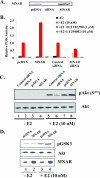
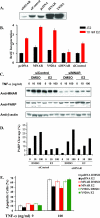
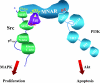
Estrogen actions are mediated by a complex interface of direct control of gene expression (the so-called "genomic action") and by regulation of cell signaling/phosphorylation cascades, referred to as the "nongenomic," or extranuclear, action. We have previously described the identification of MNAR (modulator of nongenomic action of estrogen receptor) as a novel scaffold protein that regulates estrogen receptor alpha (ERalpha) activation of cSrc. In this study, we have investigated the role of MNAR in 17beta-estradiol (E2)-induced activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway. Consistent with our previous results, a direct correlation was established between MNAR expression levels and E2-induced activation of PI3 and Akt kinases. Endogenous MNAR, ERalpha, cSrc, and p85, the regulatory subunit of PI3 kinase, interacted in MCF7 cells treated with E2. The interaction between p85 and MNAR required activation of cSrc and MNAR phosphorylation on Tyr 920. Consequently, the mutation of this tyrosine to alanine (Y920A) abrogated the interaction between MNAR and p85 and the E2-induced activation of the PI3K/Akt pathway, which was required for the E2-induced protection of MCF7 cells from apoptosis. Nonetheless, the Y920A mutant potentiated the E2-induced activation of the Src/MAPK pathway and MCF7 cell proliferation, as observed with the wild-type MNAR. These results provide new and important insights into the molecular mechanisms of E2-induced regulation of cell proliferation and apoptosis.
Figures




Similar articles
-
MNAR plays an important role in ERa activation of Src/MAPK and PI3K/Akt signaling pathways.Steroids. 2008 Oct;73(9-10):901-5. doi: 10.1016/j.steroids.2007.12.028. Epub 2008 Jan 4. Steroids. 2008. PMID: 18261753 Review.
-
Up-regulation of PI3K/Akt signaling by 17beta-estradiol through activation of estrogen receptor-alpha, but not estrogen receptor-beta, and stimulates cell growth in breast cancer cells.Biochem Biophys Res Commun. 2005 Nov 4;336(4):1221-6. doi: 10.1016/j.bbrc.2005.08.256. Biochem Biophys Res Commun. 2005. PMID: 16169518
-
Phosphatidylinositol-3-OH Kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor alpha (ERalpha) via interaction between ERalpha and PI3K.Cancer Res. 2001 Aug 15;61(16):5985-91. Cancer Res. 2001. PMID: 11507039
-
Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitric-oxide synthase activation by estrogen.J Biol Chem. 2003 Jan 24;278(4):2118-23. doi: 10.1074/jbc.M210828200. Epub 2002 Nov 12. J Biol Chem. 2003. PMID: 12431978
-
The role of adapter protein Shc in estrogen non-genomic action.Steroids. 2004 Aug;69(8-9):523-9. doi: 10.1016/j.steroids.2004.05.012. Steroids. 2004. PMID: 15288764 Review.
Cited by
-
Growth factor regulation of estrogen receptor coregulator PELP1 functions via Protein Kinase A pathway.Mol Cancer Res. 2008 May;6(5):851-61. doi: 10.1158/1541-7786.MCR-07-2030. Mol Cancer Res. 2008. PMID: 18505929 Free PMC article.
-
Oestrogen Non-Genomic Signalling is Activated in Tamoxifen-Resistant Breast Cancer.Int J Mol Sci. 2019 Jun 5;20(11):2773. doi: 10.3390/ijms20112773. Int J Mol Sci. 2019. PMID: 31195751 Free PMC article.
-
17β-Estradiol enhances breast cancer cell motility and invasion via extra-nuclear activation of actin-binding protein ezrin.PLoS One. 2011;6(7):e22439. doi: 10.1371/journal.pone.0022439. Epub 2011 Jul 26. PLoS One. 2011. PMID: 21818323 Free PMC article.
-
PELP1--a novel estrogen receptor-interacting protein.Mol Cell Endocrinol. 2008 Aug 13;290(1-2):2-7. doi: 10.1016/j.mce.2008.04.019. Epub 2008 May 13. Mol Cell Endocrinol. 2008. PMID: 18571832 Free PMC article. Review.
-
Non-genomic regulation of vascular cell function and growth by estrogen.Mol Cell Endocrinol. 2009 Sep 24;308(1-2):9-16. doi: 10.1016/j.mce.2009.03.009. Epub 2009 Mar 25. Mol Cell Endocrinol. 2009. PMID: 19549587 Free PMC article. Review.
References
-
- Barletta, F., C.-W. Wong, C. McNally, B. S. Komm, B. Katzenellenbogen, and B. J. Cheskis. 2004. Characterization of the interactions of estrogen receptor and MNAR in the activation of cSrc. Mol. Endocrinol. 18:1096-1108. - PubMed
-
- Boonyaratanakornkit, V., and D. P. Edwards. 2004. Receptor mechanisms of rapid extranuclear signalling initiated by steroid hormones. Essays Biochem. 40:105-120. - PubMed
-
- Boonyaratanakornkit, V., M. P. Scott, V. Ribon, L. Sherman, S. M. Anderson, J. L. Maller, W. T. Miller, and D. P. Edwards. 2001. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol. Cell 8:269-280. - PubMed
-
- Burow, M. E., C. B. Weldon, Y. Tang, J. A. McLachlan, and B. S. Beckman. 2001. Oestrogen-mediated suppression of tumour necrosis factor alpha-induced apoptosis in MCF-7 cells: subversion of Bcl-2 by anti-oestrogens. J. Steroid Biochem. Mol. Biol. 78:409-418. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
