Lung development and repair: contribution of the ciliated lineage
- PMID: 17194755
- PMCID: PMC1752191
- DOI: 10.1073/pnas.0610770104
Lung development and repair: contribution of the ciliated lineage
Abstract
The identity of the endogenous epithelial cells in the adult lung that are responsible for normal turnover and repair after injury is still controversial. In part, this is due to a paucity of highly specific genetic lineage tools to follow efficiently the fate of the major epithelial cell populations: the basal, secretory, ciliated, neuroendocrine, and alveolar cells. As part of a program to address this problem we have used a 1-kb FOXJ1 promoter to drive CreER in the ciliated cells of the embryonic and adult lung. Analysis of FOXJ1-GFP transgenic lungs shows that labeled cells appear in a proximal-distal pattern during embryogenesis and that the promoter drives expression in all ciliated cells. Using FOXJ1CreER adult mice, we have followed the fate of ciliated cells after epithelial injury by naphthalene or sulfur dioxide. From quantitative analysis and confocal microscopy we conclude that ciliated cells transiently change their morphology in response to lung injury but do not proliferate or transdifferentiate as part of the repair process.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
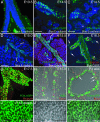



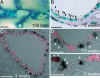



References
-
- Rawlins EL, Hogan BL. Development (Cambridge, UK) 2006;133:2455–2465. - PubMed
-
- Neuringer IP, Randell SH. Monaldi Arch Chest Dis. 2006;65:47–51. - PubMed
-
- Cardoso WV, Lu J. Development (Cambridge, UK) 2006;133:1611–1624. - PubMed
-
- Okubo T, Knoepfler PS, Eisenman RN, Hogan BL. Development (Cambridge, UK) 2005;132:1363–1374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

