Reduction of benzenoid synthesis in petunia flowers reveals multiple pathways to benzoic acid and enhancement in auxin transport
- PMID: 17194766
- PMCID: PMC1785411
- DOI: 10.1105/tpc.106.046227
Reduction of benzenoid synthesis in petunia flowers reveals multiple pathways to benzoic acid and enhancement in auxin transport
Abstract
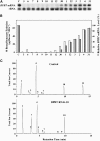
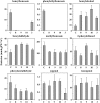
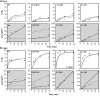
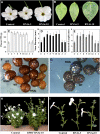
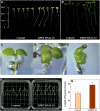
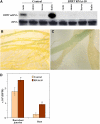
In plants, benzoic acid (BA) is believed to be synthesized from Phe through shortening of the propyl side chain by two carbons. It is hypothesized that this chain shortening occurs via either a beta-oxidative or non-beta-oxidative pathway. Previous in vivo isotope labeling and metabolic flux analysis of the benzenoid network in petunia (Petunia hybrida) flowers revealed that both pathways yield benzenoid compounds and that benzylbenzoate is an intermediate between L-Phe and BA. To test this hypothesis, we generated transgenic petunia plants in which the expression of BPBT, the gene encoding the enzyme that uses benzoyl-CoA and benzyl alcohol to make benzylbenzoate, was reduced or eliminated. Elimination of benzylbenzoate formation decreased the endogenous pool of BA and methylbenzoate emission but increased emission of benzyl alcohol and benzylaldehyde, confirming the contribution of benzylbenzoate to BA formation. Labeling experiments with 2H5-Phe revealed a dilution of isotopic abundance in most measured compounds in the dark, suggesting an alternative pathway from a precursor other than Phe, possibly phenylpyruvate. Suppression of BPBT activity also affected the overall morphology of petunia plants, resulting in larger flowers and leaves, thicker stems, and longer internodes, which was consistent with the increased auxin transport in transgenic plants. This suggests that BPBT is involved in metabolic processes in vegetative tissues as well.
Figures




References
-
- Ahmed, M.A., El-Mawla, A., and Beerhues, L. (2002). Benzoic acid biosynthesis in cell cultures of Hypericum androsaemum. Planta 214 727–733. - PubMed
-
- Baxter, I.R., Young, J.C., Armstrong, G., Foster, N., Bogenschutz, N., Cordova, T., Peer, W.A., Hazen, S.P., Murphy, A.S., and Harper, J.F. (2005). A plasma membrane H+-ATPase is required for the formation of proanthocyanidins in the seed coat endothelium of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 102 2649–2654. - PMC - PubMed
-
- Bennett, S.R.M., Alvarez, J., Bossinger, G., and Smyth, D.R. (1995). Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J. 8 505–520.
-
- Beuerle, T., and Pichersky, E. (2002). Purification and characterization of benzoate:coenzyme A ligase from Clarkia breweri. Arch. Biochem. Biophys. 400 258–264. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

