The essential nature of sphingolipids in plants as revealed by the functional identification and characterization of the Arabidopsis LCB1 subunit of serine palmitoyltransferase
- PMID: 17194770
- PMCID: PMC1785403
- DOI: 10.1105/tpc.105.040774
The essential nature of sphingolipids in plants as revealed by the functional identification and characterization of the Arabidopsis LCB1 subunit of serine palmitoyltransferase
Abstract
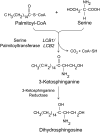
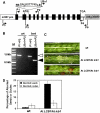
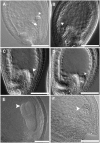
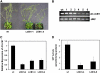
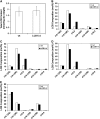
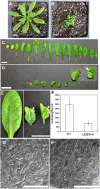
Serine palmitoyltransferase (SPT) catalyzes the first step of sphingolipid biosynthesis. In yeast and mammalian cells, SPT is a heterodimer that consists of LCB1 and LCB2 subunits, which together form the active site of this enzyme. We show that the predicted gene for Arabidopsis thaliana LCB1 encodes a genuine subunit of SPT that rescues the sphingolipid long-chain base auxotrophy of Saccharomyces cerevisiae SPT mutants when coexpressed with Arabidopsis LCB2. In addition, homozygous T-DNA insertion mutants for At LCB1 were not recoverable, but viability was restored by complementation with the wild-type At LCB1 gene. Furthermore, partial RNA interference (RNAi) suppression of At LCB1 expression was accompanied by a marked reduction in plant size that resulted primarily from reduced cell expansion. Sphingolipid content on a weight basis was not changed significantly in the RNAi suppression plants, suggesting that plants compensate for the downregulation of sphingolipid synthesis by reduced growth. At LCB1 RNAi suppression plants also displayed altered leaf morphology and increases in relative amounts of saturated sphingolipid long-chain bases. These results demonstrate that plant SPT is a heteromeric enzyme and that sphingolipids are essential components of plant cells and contribute to growth and development.
Figures


References
-
- Alexeev, D., Alexeeva, M., Baxter, R.L., Campopiano, D.J., Webster, S.P., and Sawyer, L. (1998). The crystal structure of 8-amino-7-oxononanoate synthase: A bacterial PLP-dependent, acyl-CoA-condensing enzyme. J. Mol. Biol. 284 401–419. - PubMed
-
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

