Drosophila ATR in double-strand break repair
- PMID: 17194776
- PMCID: PMC1840096
- DOI: 10.1534/genetics.106.067330
Drosophila ATR in double-strand break repair
Abstract
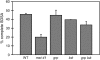
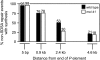
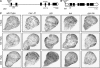
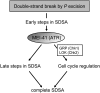
The ability of a cell to sense and respond to DNA damage is essential for genome stability. An important aspect of the response is arrest of the cell cycle, presumably to allow time for repair. Ataxia telangiectasia mutated (ATM) and ATR are essential for such cell-cycle control, but some observations suggest that they also play a direct role in DNA repair. The Drosophila ortholog of ATR, MEI-41, mediates the DNA damage-dependent G2-M checkpoint. We examined the role of MEI-41 in repair of double-strand breaks (DSBs) induced by P-element excision. We found that mei-41 mutants are defective in completing the later steps of homologous recombination repair, but have no defects in end-joining repair. We hypothesized that these repair defects are the result of loss of checkpoint control. To test this, we genetically reduced mitotic cyclin levels and also examined repair in grp (DmChk1) and lok (DmChk2) mutants. Our results suggest that a significant component of the repair defects is due to loss of MEI-41-dependent cell cycle regulation. However, this does not account for all of the defects we observed. We propose a novel role for MEI-41 in DSB repair, independent of the Chk1/Chk2-mediated checkpoint response.
Figures
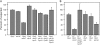

Similar articles
-
Drosophila Claspin is required for the G2 arrest that is induced by DNA replication stress but not by DNA double-strand breaks.DNA Repair (Amst). 2012 Sep 1;11(9):741-52. doi: 10.1016/j.dnarep.2012.06.007. Epub 2012 Jul 15. DNA Repair (Amst). 2012. PMID: 22796626
-
CHK1 and CHK2 are differentially involved in mismatch repair-mediated 6-thioguanine-induced cell cycle checkpoint responses.Mol Cancer Ther. 2004 Sep;3(9):1147-57. Mol Cancer Ther. 2004. PMID: 15367709
-
Artemis is a phosphorylation target of ATM and ATR and is involved in the G2/M DNA damage checkpoint response.Mol Cell Biol. 2004 Oct;24(20):9207-20. doi: 10.1128/MCB.24.20.9207-9220.2004. Mol Cell Biol. 2004. PMID: 15456891 Free PMC article.
-
The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer.Adv Cancer Res. 2010;108:73-112. doi: 10.1016/B978-0-12-380888-2.00003-0. Adv Cancer Res. 2010. PMID: 21034966 Review.
-
Cell-cycle checkpoint kinases: checking in on the cell cycle.Curr Opin Cell Biol. 2000 Dec;12(6):697-704. doi: 10.1016/s0955-0674(00)00154-x. Curr Opin Cell Biol. 2000. PMID: 11063934 Review.
Cited by
-
Multiple functions of Drosophila BLM helicase in maintenance of genome stability.Genetics. 2007 Aug;176(4):1979-92. doi: 10.1534/genetics.106.070052. Epub 2007 May 16. Genetics. 2007. PMID: 17507683 Free PMC article.
-
Loss of Drosophila Mei-41/ATR Alters Meiotic Crossover Patterning.Genetics. 2018 Feb;208(2):579-588. doi: 10.1534/genetics.117.300634. Epub 2017 Dec 15. Genetics. 2018. PMID: 29247012 Free PMC article.
-
Reducing DNA polymerase alpha in the absence of Drosophila ATR leads to P53-dependent apoptosis and developmental defects.Genetics. 2007 Jul;176(3):1441-51. doi: 10.1534/genetics.107.073635. Epub 2007 May 4. Genetics. 2007. PMID: 17483406 Free PMC article.
-
Direct Binding to Replication Protein A (RPA)-coated Single-stranded DNA Allows Recruitment of the ATR Activator TopBP1 to Sites of DNA Damage.J Biol Chem. 2016 Jun 17;291(25):13124-31. doi: 10.1074/jbc.M116.729194. Epub 2016 Apr 26. J Biol Chem. 2016. PMID: 27129245 Free PMC article.
-
Mechanisms of recombination between diverged sequences in wild-type and BLM-deficient mouse and human cells.Mol Cell Biol. 2010 Apr;30(8):1887-97. doi: 10.1128/MCB.01553-09. Epub 2010 Feb 12. Mol Cell Biol. 2010. PMID: 20154148 Free PMC article.
References
-
- Abraham, R. T., 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15: 2177–2196. - PubMed
-
- Adams, M. D., M. McVey and J. Sekelsky, 2003. Drosophila BLM in double-strand break repair by synthesis-dependent strand annealing. Science 299: 265–267. - PubMed
-
- Banga, S. S., A. Velazquez and J. B. Boyd, 1991. P transposition in Drosophila provides a new tool for analyzing postreplication repair and double-strand break repair. Mutat. Res. 255: 79–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

