Efficacy of orally administered T-705 on lethal avian influenza A (H5N1) virus infections in mice
- PMID: 17194832
- PMCID: PMC1803113
- DOI: 10.1128/AAC.01051-06
Efficacy of orally administered T-705 on lethal avian influenza A (H5N1) virus infections in mice
Abstract
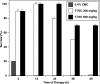
T-705 (6-fluoro-3-hydroxy-2-pyrazinecarboxamide) was inhibitory to four strains of avian H5N1 influenza virus in MDCK cells, with the 90% effective concentrations ranging from 1.3 to 7.7 microM, as determined by a virus yield reduction assay. The efficacy was less than that exerted by oseltamivir carboxylate or zanamivir but was greater than that exerted by ribavirin. Experiments with mice lethally infected with influenza A/Duck/MN/1525/81 (H5N1) virus showed that T-705 administered per os once, twice, or four times daily for 5 days beginning 1 h after virus exposure was highly inhibitory to the infection. Dosages from 30 to 300 mg/kg of body weight/day were well tolerated; each prevented death, lessened the decline of arterial oxygen saturation (SaO(2)), and inhibited lung consolidation and lung virus titers. Dosages from 30 to 300 mg/kg/day administered once or twice daily also significantly prevented the death of the mice. Oseltamivir (20 mg/kg/day), administered per os twice daily for 5 days, was tested in parallel in two experiments; it was only weakly effective against the infection. The four-times-daily T-705 treatments at 300 mg/kg/day could be delayed until 96 h after virus exposure and still significantly inhibit the infection. Single T-705 treatments administered up to 60 h after virus exposure also prevented death and the decline of SaO(2). Characterization of the pathogenesis of the duck influenza H5N1 virus used in these studies was undertaken; although the virus was highly pathogenic to mice, it was less neurotropic than has been described for clinical isolates of the H5N1 virus. These data indicate that T-705 may be useful for the treatment of avian influenza virus infections.
Figures

Similar articles
-
Comparison of efficacies of RWJ-270201, zanamivir, and oseltamivir against H5N1, H9N2, and other avian influenza viruses.Antimicrob Agents Chemother. 2001 Oct;45(10):2723-32. doi: 10.1128/AAC.45.10.2723-2732.2001. Antimicrob Agents Chemother. 2001. PMID: 11557461 Free PMC article.
-
Effects of the combination of favipiravir (T-705) and oseltamivir on influenza A virus infections in mice.Antimicrob Agents Chemother. 2010 Jan;54(1):126-33. doi: 10.1128/AAC.00933-09. Epub 2009 Nov 9. Antimicrob Agents Chemother. 2010. PMID: 19901093 Free PMC article.
-
In vitro and in vivo efficacy of fluorodeoxycytidine analogs against highly pathogenic avian influenza H5N1, seasonal, and pandemic H1N1 virus infections.Antiviral Res. 2011 Nov;92(2):329-40. doi: 10.1016/j.antiviral.2011.09.001. Epub 2011 Sep 8. Antiviral Res. 2011. PMID: 21925541 Free PMC article.
-
Treatment options for H5N1: lessons learned from the H1N1 pandemic.Postgrad Med. 2010 Sep;122(5):134-41. doi: 10.3810/pgm.2010.09.2210. Postgrad Med. 2010. PMID: 20861597 Review.
-
Laninamivir octanoate: a new long-acting neuraminidase inhibitor for the treatment of influenza.Expert Rev Anti Infect Ther. 2011 Oct;9(10):851-7. doi: 10.1586/eri.11.112. Expert Rev Anti Infect Ther. 2011. PMID: 21973296 Review.
Cited by
-
The ambiguous base-pairing and high substrate efficiency of T-705 (Favipiravir) Ribofuranosyl 5'-triphosphate towards influenza A virus polymerase.PLoS One. 2013 Jul 10;8(7):e68347. doi: 10.1371/journal.pone.0068347. Print 2013. PLoS One. 2013. PMID: 23874596 Free PMC article.
-
Antivirals for influenza: strategies for use in pediatrics.Paediatr Drugs. 2010 Oct 1;12(5):285-99. doi: 10.2165/11532530-000000000-00000. Paediatr Drugs. 2010. PMID: 20799758 Review.
-
Identification and Development of Therapeutics for COVID-19.mSystems. 2021 Dec 21;6(6):e0023321. doi: 10.1128/mSystems.00233-21. Epub 2021 Nov 2. mSystems. 2021. PMID: 34726496 Free PMC article.
-
Favipiravir (T-705), a novel viral RNA polymerase inhibitor.Antiviral Res. 2013 Nov;100(2):446-54. doi: 10.1016/j.antiviral.2013.09.015. Epub 2013 Sep 29. Antiviral Res. 2013. PMID: 24084488 Free PMC article. Review.
-
Antiviral efficacy of favipiravir against two prominent etiological agents of hantavirus pulmonary syndrome.Antimicrob Agents Chemother. 2013 Oct;57(10):4673-80. doi: 10.1128/AAC.00886-13. Epub 2013 Jul 15. Antimicrob Agents Chemother. 2013. PMID: 23856782 Free PMC article.
References
-
- Baigent, S. J., and J. W. McCauley. 2003. Influenza type A in humans, mammals and birds: determinants of virus virulence, host-range and interspecies transmission. Bioessays 25:657-671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

