Pten (phosphatase and tensin homologue gene) haploinsufficiency promotes insulin hypersensitivity
- PMID: 17195063
- PMCID: PMC1781097
- DOI: 10.1007/s00125-006-0531-x
Pten (phosphatase and tensin homologue gene) haploinsufficiency promotes insulin hypersensitivity
Abstract
Aims/hypothesis: Insulin controls glucose metabolism via multiple signalling pathways, including the phosphatidylinositol 3-kinase (PI3K) pathway in muscle and adipose tissue. The protein/lipid phosphatase Pten (phosphatase and tensin homologue deleted on chromosome 10) attenuates PI3K signalling by dephosphorylating the phosphatidylinositol 3,4,5-trisphosphate generated by PI3K. The current study was aimed at investigating the effect of haploinsufficiency for Pten on insulin-stimulated glucose uptake.
Materials and methods: Insulin sensitivity in Pten heterozygous (Pten(+/-)) mice was investigated in i.p. insulin challenge and glucose tolerance tests. Glucose uptake was monitored in vitro in primary cultures of myocytes from Pten(+/-) mice, and in vivo by positron emission tomography. The phosphorylation status of protein kinase B (PKB/Akt), a downstream signalling protein in the PI3K pathway, and glycogen synthase kinase 3beta (GSK3beta), a substrate of PKB/Akt, was determined by western immunoblotting.
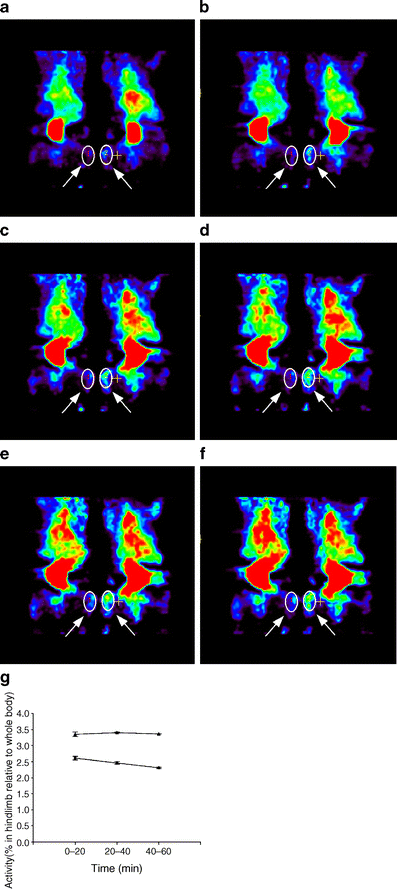
Results: Following i.p. insulin challenge, blood glucose levels in Pten(+/-) mice remained depressed for up to 120 min, whereas glucose levels in wild-type mice began to recover after approximately 30 min. After glucose challenge, blood glucose returned to normal about twice as rapidly in Pten(+/-) mice. Enhanced glucose uptake was observed both in Pten(+/-) myocytes and in skeletal muscle of Pten(+/-) mice by PET. PKB and GSK3beta phosphorylation was enhanced and prolonged in Pten(+/-) myocytes.
Conclusions/interpretation: Pten is a key negative regulator of insulin-stimulated glucose uptake in vitro and in vivo. The partial reduction of Pten due to Pten haploinsufficiency is enough to elicit enhanced insulin sensitivity and glucose tolerance in Pten(+/-) mice.
Figures




Comment in
-
PTEN targeting: the search for novel insulin sensitisers provides new insight into obesity research.Diabetologia. 2007 Feb;50(2):247-9. doi: 10.1007/s00125-006-0547-2. Diabetologia. 2007. PMID: 17136390 No abstract available.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S0960-9822(02)00777-7', 'is_inner': False, 'url': 'https://doi.org/10.1016/s0960-9822(02)00777-7'}, {'type': 'PubMed', 'value': '11937037', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11937037/'}]}
- Lizcano JM, Alessi DR (2002) The insulin signalling pathway. Curr Biol 12:R236–R238 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1074/jbc.273.22.13375', 'is_inner': False, 'url': 'https://doi.org/10.1074/jbc.273.22.13375'}, {'type': 'PubMed', 'value': '9593664', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9593664/'}]}
- Maehama T, Dixon JE (1998) The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273:13375–13378 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1073/pnas.95.23.13513', 'is_inner': False, 'url': 'https://doi.org/10.1073/pnas.95.23.13513'}, {'type': 'PMC', 'value': 'PMC24850', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC24850/'}, {'type': 'PubMed', 'value': '9811831', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9811831/'}]}
- Myers MP, Pass I, Batty IH et al (1998) The lipid phosphatase activity of PTEN is critical for its tumor suppressor function. Proc Natl Acad Sci USA 95:13513–13518 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1126/science.275.5308.1943', 'is_inner': False, 'url': 'https://doi.org/10.1126/science.275.5308.1943'}, {'type': 'PubMed', 'value': '9072974', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9072974/'}]}
- Li J, Yen C, Liaw D et al (1997) PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275:1943–1947 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1038/ng0497-356', 'is_inner': False, 'url': 'https://doi.org/10.1038/ng0497-356'}, {'type': 'PubMed', 'value': '9090379', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9090379/'}]}
- Steck PA, Pershouse MA, Jasser SA et al (1997) Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 15:356–362 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

