A comparative analysis of the sugar phosphate cyclase superfamily involved in primary and secondary metabolism
- PMID: 17195255
- PMCID: PMC3127856
- DOI: 10.1002/cbic.200600446
A comparative analysis of the sugar phosphate cyclase superfamily involved in primary and secondary metabolism
Abstract
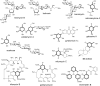
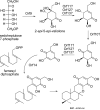
Sugar phosphate cyclases (SPCs) catalyze the cyclization of sugar phosphates to produce a variety of cyclitol intermediates that serve as the building blocks of many primary metabolites, for example, aromatic amino acids, and clinically relevant secondary metabolites, for example, aminocyclitol/aminoglycoside and ansamycin antibiotics. Feeding experiments with isotopically labeled cyclitols revealed that cetoniacytone A, a unique C(7)N-aminocyclitol antibiotic isolated from an insect endophytic Actinomyces sp., is derived from 2-epi-5-epi-valiolone, a product of SPC. By using heterologous probes from the 2-epi-5-epi-valiolone synthase class of SPCs, an SPC homologue gene, cetA, was isolated from the cetoniacytone producer. cetA is closely related to BE-orf9 found in the BE-40644 biosynthetic gene cluster from Actinoplanes sp. strain A40644. Recombinant expression of cetA and BE-orf9 and biochemical characterization of the gene products confirmed their function as 2-epi-5-epi-valiolone synthases. Further phylogenetic analysis of SPC sequences revealed a new clade of SPCs that might regulate the biosynthesis of a novel set of secondary metabolites.
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

