Modularity and dynamics of cellular networks
- PMID: 17196032
- PMCID: PMC1761653
- DOI: 10.1371/journal.pcbi.0020174
Modularity and dynamics of cellular networks
Conflict of interest statement
Figures




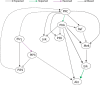



References
-
- Lockhart DJ, Winzeler EA. Genomics, gene expression and DNA arrays. Nature. 2000;405:827–836. - PubMed
-
- Walhout AJ, Vidal M. Protein interaction maps for model organisms. Nat Rev Mol Cell Biol. 2001;2:55–62. - PubMed
-
- Lee TI, Rinaldi NJ, Robert F, Odom DT, Bar-Joseph Z, et al. Transcriptional regulatory networks in Saccharomyces cerevisiae . Science. 2002;298:799–804. - PubMed
-
- Drewes G, Bouwmeester T. Global approaches to protein–protein interactions. Curr Opin Cell Biol. 2003;15:199–205. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

