Improved outcomes in patients with chronic obstructive pulmonary disease treated with salmeterol compared with placebo/usual therapy: results of a meta-analysis
- PMID: 17196106
- PMCID: PMC1779294
- DOI: 10.1186/1465-9921-7-147
Improved outcomes in patients with chronic obstructive pulmonary disease treated with salmeterol compared with placebo/usual therapy: results of a meta-analysis
Abstract
Background: Several studies have demonstrated that long-acting beta2-agonists such as salmeterol are beneficial in chronic obstructive pulmonary disease (COPD). A meta-analysis was therefore conducted to review studies in COPD to provide pooled estimates of the effect of salmeterol 50 mcg taken twice daily in addition to usual therapy on several clinically relevant endpoints, when compared with placebo/usual therapy.
Methods: An extensive search of literature and clinical trial databases was conducted using the terms salmeterol, COPD, chronic, obstructive, bronchitis and emphysema. Nine randomized, double-blind, parallel-group, placebo-controlled trials of > or =12 week duration with salmeterol 50 mcg bid treatment in COPD were included (>3500 patients), with a further 14 trials excluded due to study design or reporting timelines. All patients were included, and a sub-group of subjects (84%) with poorly reversible COPD were considered separately. Statistical testing was carried out at the 5% level, except for interaction testing which was carried out at the 10% level.
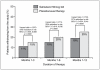
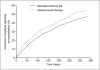
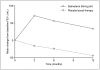
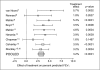
Results: Patients treated with salmeterol over 12 months were less likely to withdraw early from the studies (19% patients compared with 25% on their current usual therapy, p < 0.001), less likely to suffer a moderate/severe exacerbation (34% compared with 39%, p < 0.0001) and had a greater increase in average FEV1 (73 mL difference vs placebo/usual therapy, p < 0.0001). Similar differences were found at 3 and 6 months. At all time points, more patients experienced an improvement in health status and also a greater change with salmeterol than with placebo/usual therapy (p < 0.002). There was no evidence of tachyphylaxis to salmeterol over 12 months.
Conclusion: The meta-analysis confirmed clinically and statistically significant, sustained and consistent superiority of salmeterol 50 mcg bid over placebo/usual therapy on a broad range of outcome measures.
Figures

References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) Publication Number 2701. National Institutes of Health, National Heart Lung and Blood Institute; 2001. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease NHLBI/WHO workshop report.
-
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–1422. - PubMed
-
- Wildman M, Groves J, Walia S, Stableforth D, Ayres J. Hospitalised COPD exacerbations: survival and univariate outcome predictors, 36-month follow-up [abstract] Am J Respir Crit Care Med. 2002;165(2 pt 2):A272.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

