Rescue of odontogenesis in Dmp1-deficient mice by targeted re-expression of DMP1 reveals roles for DMP1 in early odontogenesis and dentin apposition in vivo
- PMID: 17196192
- PMCID: PMC2059935
- DOI: 10.1016/j.ydbio.2006.11.001
Rescue of odontogenesis in Dmp1-deficient mice by targeted re-expression of DMP1 reveals roles for DMP1 in early odontogenesis and dentin apposition in vivo
Abstract
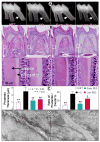
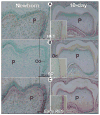
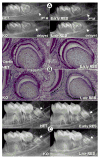
Dentin matrix protein 1 (DMP1) is expressed in both pulp and odontoblast cells and deletion of the Dmp1 gene leads to defects in odontogenesis and mineralization. The goals of this study were to examine how DMP1 controls dentin mineralization and odontogenesis in vivo. Fluorochrome labeling of dentin in Dmp1-null mice showed a diffuse labeling pattern with a 3-fold reduction in dentin appositional rate compared to controls. Deletion of DMP1 was also associated with abnormalities in the dentinal tubule system and delayed formation of the third molar. Unlike the mineralization defect in Vitamin D receptor-null mice, the mineralization defect in Dmp1-null mice was not rescued by a high calcium and phosphate diet, suggesting a different effect of DMP1 on mineralization. Re-expression of Dmp1 in early and late odontoblasts under control of the Col1a1 promoter rescued the defects in mineralization as well as the defects in the dentinal tubules and third molar development. In contrast, re-expression of Dmp1 in mature odontoblasts, using the Dspp promoter, produced only a partial rescue of the mineralization defects. These data suggest that DMP1 is a key regulator of odontoblast differentiation, formation of the dentin tubular system and mineralization and its expression is required in both early and late odontoblasts for normal odontogenesis to proceed.
Figures
Similar articles
-
Dentin matrix protein 1 and phosphate homeostasis are critical for postnatal pulp, dentin and enamel formation.Int J Oral Sci. 2012 Dec;4(4):189-95. doi: 10.1038/ijos.2012.69. Epub 2012 Dec 21. Int J Oral Sci. 2012. PMID: 23258378 Free PMC article.
-
The rescue of dentin matrix protein 1 (DMP1)-deficient tooth defects by the transgenic expression of dentin sialophosphoprotein (DSPP) indicates that DSPP is a downstream effector molecule of DMP1 in dentinogenesis.J Biol Chem. 2013 Mar 8;288(10):7204-14. doi: 10.1074/jbc.M112.445775. Epub 2013 Jan 24. J Biol Chem. 2013. PMID: 23349460 Free PMC article.
-
Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo.J Bone Miner Res. 1997 Dec;12(12):2040-9. doi: 10.1359/jbmr.1997.12.12.2040. J Bone Miner Res. 1997. PMID: 9421236
-
Extracellular matrix proteins of dentine.Ciba Found Symp. 1997;205:107-15; discussion 115-7. doi: 10.1002/9780470515303.ch8. Ciba Found Symp. 1997. PMID: 9189620 Review.
-
Odontoblasts: Specialized hard-tissue-forming cells in the dentin-pulp complex.Congenit Anom (Kyoto). 2016 Jul;56(4):144-53. doi: 10.1111/cga.12169. Congenit Anom (Kyoto). 2016. PMID: 27131345 Review.
Cited by
-
A novel chitin binding crayfish molar tooth protein with elasticity properties.PLoS One. 2015 May 26;10(5):e0127871. doi: 10.1371/journal.pone.0127871. eCollection 2015. PLoS One. 2015. PMID: 26010981 Free PMC article.
-
Skeletal secretion of FGF-23 regulates phosphate and vitamin D metabolism.Nat Rev Endocrinol. 2012 Jan 17;8(5):276-86. doi: 10.1038/nrendo.2011.218. Nat Rev Endocrinol. 2012. PMID: 22249518 Free PMC article. Review.
-
Disruption of Smad4 in odontoblasts causes multiple keratocystic odontogenic tumors and tooth malformation in mice.Mol Cell Biol. 2009 Nov;29(21):5941-51. doi: 10.1128/MCB.00706-09. Epub 2009 Aug 24. Mol Cell Biol. 2009. PMID: 19703995 Free PMC article.
-
BDNF/TrkB Is a Crucial Regulator in the Inflammation-Mediated Odontoblastic Differentiation of Dental Pulp Stem Cells.Cells. 2023 Jul 14;12(14):1851. doi: 10.3390/cells12141851. Cells. 2023. PMID: 37508514 Free PMC article.
-
Endocrine functions of bone in mineral metabolism regulation.J Clin Invest. 2008 Dec;118(12):3820-8. doi: 10.1172/JCI36479. Epub 2008 Dec 1. J Clin Invest. 2008. PMID: 19033649 Free PMC article. Review.
References
-
- Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, Demay MB. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology. 1999;140:4982–7. - PubMed
-
- Braut A, Kalajzic I, Kalajzic Z, Rowe DW, Kollar EJ, Mina M. Col1a1-GFP transgene expression in developing incisors. Connect Tissue Res. 2002;43:216–9. - PubMed
-
- Butler WT, Brunn JC, Qin C, McKee MD. Extracellular matrix proteins and the dynamics of dentin formation. Connect Tissue Res. 2002;43:301–7. - PubMed
-
- Chen J, Shapiro HS, Sodek J. Development expression of bone sialoprotein mRNA in rat mineralized connective tissues. J Bone Miner Res. 1992;7:987–97. - PubMed
-
- D’Souza RN, Cavender A, Sunavala G, Alvarez J, Ohshima T, Kulkarni AB, MacDougall M. Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. Journal of Bone & Mineral Research. 1997;12:2040–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

