Tissue strain amplification at the osteocyte lacuna: a microstructural finite element analysis
- PMID: 17196968
- PMCID: PMC2094105
- DOI: 10.1016/j.jbiomech.2006.10.040
Tissue strain amplification at the osteocyte lacuna: a microstructural finite element analysis
Abstract
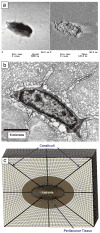
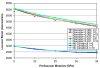
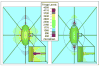
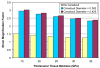
A parametric finite element model of an osteocyte lacuna was developed to predict the microstructural response of the lacuna to imposed macroscopic strains. The model is composed of an osteocyte lacuna, a region of perilacunar tissue, canaliculi, and the surrounding bone tissue. A total of 45 different simulations were modeled with varying canalicular diameters, perilacunar tissue material moduli, and perilacunar tissue thicknesses. Maximum strain increased with a decrease in perilacunar tissue modulus and decreased with an increase in perilacunar tissue modulus, regardless of the thickness of the perilacunar region. An increase in the predicted maximum strain was observed with an increase in canalicular diameter from 0.362 to 0.421 microm. In response to the macroscopic application of strain, canalicular diameters increased 0.8% to over 1.0% depending on the perilacunar tissue modulus. Strain magnification factors of over 3 were predicted. However, varying the size of the perilacunar tissue region had no effect on the predicted perilacunar tissue strain. These results indicate that the application of average macroscopic strains similar to strain levels measured in vivo can result in significantly greater perilacunar tissue strains and canaliculi deformations. A decrease in the perilacunar tissue modulus amplifies the perilacunar tissue strain and canaliculi deformation while an increase in the local perilacunar tissue modulus attenuates this effect.
Figures



References
-
- Aarden EM, Nijweide PJ, van der Plas A, Alblas MJ, Mackie EJ, Horton MA, Helfrich MH. Adhesive properties of isolated chick osteocytes in vitro. Bone. 1996;18:305–313. - PubMed
-
- Alexopolous LG, Setton LA, Guilak F. The biomechanical role of the chondrocyte pericellular matrix in articular cartilage. Acta Biomaterialia. 2005;1:317–325. - PubMed
-
- Bacabac RG, Smit TH, Mullender MG, Dijcks SJ, Van Loon JJ, Klein-Nulend J. Nitric oxide production by bone cells is fluid shear stress rate dependent. Biochemical and Biophysical Research Communications. 2004;315:823–829. - PubMed
-
- Baylink DJ, Wergedal JE. Bone formation by osteocytes. American Journal of Physiology. 1971;221:669–678. - PubMed
-
- Beno T, Yoon YJ, Cowin SC, Fritton SP. Estimation of bone permeability using accurate microstructural measurements. Journal of Biomechanics. 2006 in press. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

