par genes and the pathology of chromosome loss in Vibrio cholerae
- PMID: 17197419
- PMCID: PMC1760642
- DOI: 10.1073/pnas.0608341104
par genes and the pathology of chromosome loss in Vibrio cholerae
Abstract
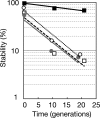
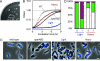
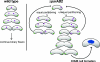
The causes and consequences of chromosome loss in bacteria with multiple chromosomes are unknown. Vibrio cholerae, the causative agent of the severe diarrheal disease cholera, has two circular chromosomes. Like many other bacterial chromosomes, both V. cholerae chromosomes contain homologues of plasmid partitioning (par) genes. In plasmids, par genes act to segregate plasmid molecules to daughter cells and thereby ensure plasmid maintenance; however, the contribution of par genes to chromosome segregation is not clear. Here, we show that the chromosome II parAB2 genes are essential for the segregation of chromosome II but not chromosome I. In a parAB2 deletion mutant, chromosome II is mislocalized and frequently fails to segregate, yielding cells with only chromosome I. These cells divide once; their progeny are not viable. Instead, chromosome II-deficient cells undergo dramatic cell enlargement, nucleoid condensation and degradation, and loss of membrane integrity. The highly consistent nature of these cytologic changes suggests that prokaryotes, like eukaryotes, may possess characteristic death pathways.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ogura T, Hiraga S. Cell. 1983;32:351–360. - PubMed
-
- Austin S, Abeles A. J Mol Biol. 1983;169:373–387. - PubMed
-
- Niki H, Hiraga S. Cell. 1997;90:951–957. - PubMed
-
- Li Y, Dabrazhynetskaya A, Youngren B, Austin S. Mol Microbiol. 2004;53:93–102. - PubMed
-
- Ebersbach G, Gerdes K. Annu Rev Genet. 2005;39:453–479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

