Inhibition of cytokine signaling in human retinal endothelial cells through modification of caveolae/lipid rafts by docosahexaenoic acid
- PMID: 17197511
- PMCID: PMC1975816
- DOI: 10.1167/iovs.06-0619
Inhibition of cytokine signaling in human retinal endothelial cells through modification of caveolae/lipid rafts by docosahexaenoic acid
Abstract
Purpose: Docosahexaenoic acid (DHA(22:6,n3)) is the principal n3 polyunsaturated fatty acid (PUFA) in the retina. The authors previously demonstrated that DHA(22:6,n3) inhibited cytokine-induced adhesion molecule expression in primary human retinal vascular endothelial (hRVE) cells, the target tissue affected by diabetic retinopathy. Despite the importance of vascular inflammation in diabetic retinopathy, the mechanisms underlying anti-inflammatory effects of DHA(22:6,n3) in vascular endothelial cells are not understood. In this study the authors address the hypothesis that DHA(22:6,n3) acts through modifying lipid composition of caveolae/lipid rafts, thereby changing the outcome of important signaling events in these specialized plasma membrane microdomains.
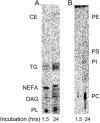
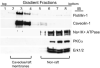
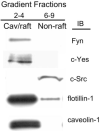
Methods: hRVE cells were cultured in the presence or absence of DHA(22:6,n3). Isolated caveolae/lipid raft-enriched detergent-resistant membrane domains were prepared using sucrose gradient ultracentrifugation. Fatty acid composition and cholesterol content of caveolae/lipid rafts before and after treatment were measured by HPLC. The expression of Src family kinases was assayed by Western blotting and immunohistochemistry.
Results: Disruption of the caveolae/lipid raft structure with a cholesterol-depleting agent, methyl-cyclodextrin (MCD), diminished cytokine-induced signaling in hRVE cells. Growth of hRVE cells in media enriched in DHA(22:6,n3) resulted in significant incorporation of DHA(22:6,n3) into the major phospholipids of caveolae/lipid rafts, causing an increase in the unsaturation index in the membrane microdomain. DHA(22:6,n3) enrichment in the caveolae/raft was accompanied by a 70% depletion of cholesterol from caveolae/lipid rafts and displacement of the SFK, Fyn, and c-Yes from caveolae/lipid rafts. Adding water-soluble cholesterol to DHA(22:6,n3)-treated cells replenished cholesterol in caveolae/lipid rafts and reversed the effect of DHA(22:6,n3) on cytokine-induced signaling.
Conclusions: Incorporation of DHA(22:6,n3) into fatty acyl chains of phospholipids in caveolae/lipid rafts, followed by cholesterol depletion and displacement of important signaling molecules, provides a potential mechanism for anti-inflammatory effect of DHA(22:6,n3) in hRVE cells.
Figures






Similar articles
-
Polyunsaturated docosahexaenoic acid suppresses oxidative stress induced endothelial cell calcium influx by altering lipid composition in membrane caveolar rafts.Prostaglandins Leukot Essent Fatty Acids. 2010 Jul;83(1):37-43. doi: 10.1016/j.plefa.2010.02.002. Epub 2010 Mar 4. Prostaglandins Leukot Essent Fatty Acids. 2010. PMID: 20206488
-
Anti-inflammatory effect of docosahexaenoic acid on cytokine-induced adhesion molecule expression in human retinal vascular endothelial cells.Invest Ophthalmol Vis Sci. 2005 Nov;46(11):4342-7. doi: 10.1167/iovs.05-0601. Invest Ophthalmol Vis Sci. 2005. PMID: 16249517 Free PMC article.
-
Docosahexaenoic Acid Controls Pulmonary Macrophage Lipid Raft Size and Inflammation.J Nutr. 2024 Jun;154(6):1945-1958. doi: 10.1016/j.tjnut.2024.04.006. Epub 2024 Apr 4. J Nutr. 2024. PMID: 38582385 Free PMC article.
-
Docosahexaenoic acid domains: the ultimate non-raft membrane domain.Chem Phys Lipids. 2008 May;153(1):57-63. doi: 10.1016/j.chemphyslip.2008.02.010. Epub 2008 Feb 23. Chem Phys Lipids. 2008. PMID: 18343224 Review.
-
Rafting on the Plasma Membrane: Lipid Rafts in Signaling and Disease.Adv Exp Med Biol. 2023;1436:87-108. doi: 10.1007/5584_2022_759. Adv Exp Med Biol. 2023. PMID: 36648750 Review.
Cited by
-
Assessment of Inner Blood-Retinal Barrier: Animal Models and Methods.Cells. 2023 Oct 12;12(20):2443. doi: 10.3390/cells12202443. Cells. 2023. PMID: 37887287 Free PMC article. Review.
-
ω-3 and ω-6 long-chain PUFAs and their enzymatic metabolites in neovascular eye diseases.Am J Clin Nutr. 2017 Jul;106(1):16-26. doi: 10.3945/ajcn.117.153825. Epub 2017 May 17. Am J Clin Nutr. 2017. PMID: 28515072 Free PMC article. Review.
-
DHA inhibited AGEs-induced retinal microglia activation via suppression of the PPARγ/NFκB pathway and reduction of signal transducers in the AGEs/RAGE axis recruitment into lipid rafts.Neurochem Res. 2015 Apr;40(4):713-22. doi: 10.1007/s11064-015-1517-1. Epub 2015 Jan 18. Neurochem Res. 2015. PMID: 25596942
-
Docosahexaenoic acid improves the nitroso-redox balance and reduces VEGF-mediated angiogenic signaling in microvascular endothelial cells.Invest Ophthalmol Vis Sci. 2010 Dec;51(12):6815-25. doi: 10.1167/iovs.10-5339. Epub 2010 Aug 11. Invest Ophthalmol Vis Sci. 2010. PMID: 20702831 Free PMC article.
-
Nano-scale resolution of native retinal rod disk membranes reveals differences in lipid composition.J Cell Biol. 2021 Aug 2;220(8):e202101063. doi: 10.1083/jcb.202101063. Epub 2021 Jun 16. J Cell Biol. 2021. PMID: 34132745 Free PMC article.
References
-
- Lee TH, Hoover RL, Williams JD, et al. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N Engl J Med. 1985;312:1217–1224. - PubMed
-
- Trebble T, Arden NK, Stroud MA, et al. Inhibition of tumour necrosis factor-alpha and interleukin 6 production by mononuclear cells following dietary fish-oil supplementation in healthy men and response to antioxidant co-supplementation. Br J Nutr. 2003;90:405–412. - PubMed
-
- Diaz O, Berquand A, Dubois M, et al. The mechanism of docosahexaenoic acid induced phospholipase D activation in human lymphocytes involves exclusion of the enzyme from lipid rafts. J Biol Chem. 2002;277:39368–39378. - PubMed
-
- Fan YY, Ly LH, Barhoumi R, McMurray DN, Chapkin RS. Dietary docosahexaenoic acid suppresses T cell protein kinase C-theta lipid raft recruitment and IL-2 production. J Immunol. 2004;173:6151–6160. - PubMed
-
- Fan YY, McMurray DN, Ly LH, Chapkin RS. Dietary (n-3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J Nutr. 2003;133:1913–1920. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

