Importance of the 5 S rRNA-binding ribosomal proteins for cell viability and translation in Escherichia coli
- PMID: 17198710
- PMCID: PMC1939977
- DOI: 10.1016/j.jmb.2006.11.097
Importance of the 5 S rRNA-binding ribosomal proteins for cell viability and translation in Escherichia coli
Abstract
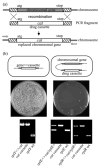
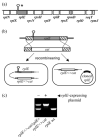
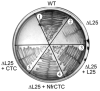
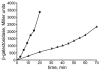
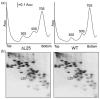
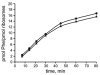
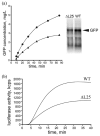
A specific complex of 5 S rRNA and several ribosomal proteins is an integral part of ribosomes in all living organisms. Here we studied the importance of Escherichia coli genes rplE, rplR and rplY, encoding 5 S rRNA-binding ribosomal proteins L5, L18 and L25, respectively, for cell growth, viability and translation. Using recombineering to create gene replacements in the E. coli chromosome, it was shown that rplE and rplR are essential for cell viability, whereas cells deleted for rplY are viable, but grow noticeably slower than the parental strain. The slow growth of these L25-defective cells can be stimulated by a plasmid expressing the rplY gene and also by a plasmid bearing the gene for homologous to L25 general stress protein CTC from Bacillus subtilis. The rplY mutant ribosomes are physically normal and contain all ribosomal proteins except L25. The ribosomes from L25-defective and parental cells translate in vitro at the same rate either poly(U) or natural mRNA. The difference observed was that the mutant ribosomes synthesized less natural polypeptide, compared to wild-type ribosomes both in vivo and in vitro. We speculate that the defect is at the ribosome recycling step.
Figures
Similar articles
-
Regulation of the rplY gene encoding 5S rRNA binding protein L25 in Escherichia coli and related bacteria.RNA. 2015 May;21(5):851-61. doi: 10.1261/rna.047381.114. Epub 2015 Mar 6. RNA. 2015. PMID: 25749694 Free PMC article.
-
Mutant forms of Escherichia coli protein L25 unable to bind to 5S rRNA are incorporated efficiently into the ribosome in vivo.Biochemistry (Mosc). 2014 Aug;79(8):826-35. doi: 10.1134/S0006297914080112. Biochemistry (Mosc). 2014. PMID: 25365493
-
Construction and functional analysis of ribosomal 5S RNA from Escherichia coli with single base changes in the ribosomal protein binding sites.Biol Chem Hoppe Seyler. 1986 Aug;367(8):769-80. doi: 10.1515/bchm3.1986.367.2.769. Biol Chem Hoppe Seyler. 1986. PMID: 2429677
-
5S rRNA and ribosome.Biochemistry (Mosc). 2011 Dec;76(13):1450-64. doi: 10.1134/S0006297911130062. Biochemistry (Mosc). 2011. PMID: 22339598 Review.
-
Assembly of bacterial ribosomes.Annu Rev Biochem. 2011;80:501-26. doi: 10.1146/annurev-biochem-062608-160432. Annu Rev Biochem. 2011. PMID: 21529161 Review.
Cited by
-
Simplification of Ribosomes in Bacteria with Tiny Genomes.Mol Biol Evol. 2021 Jan 4;38(1):58-66. doi: 10.1093/molbev/msaa184. Mol Biol Evol. 2021. PMID: 32681797 Free PMC article.
-
5SRNAdb: an information resource for 5S ribosomal RNAs.Nucleic Acids Res. 2016 Jan 4;44(D1):D180-3. doi: 10.1093/nar/gkv1081. Epub 2015 Oct 20. Nucleic Acids Res. 2016. PMID: 26490961 Free PMC article.
-
Eukaryotic 5S rRNA biogenesis.Wiley Interdiscip Rev RNA. 2011 Jul-Aug;2(4):523-33. doi: 10.1002/wrna.74. Epub 2011 Feb 25. Wiley Interdiscip Rev RNA. 2011. PMID: 21957041 Free PMC article. Review.
-
Proteomic analysis of Salmonella enterica serovar Enteritidis following propionate adaptation.BMC Microbiol. 2010 Sep 28;10:249. doi: 10.1186/1471-2180-10-249. BMC Microbiol. 2010. PMID: 20920181 Free PMC article.
-
Adaptive Responses of Pseudomonas aeruginosa to Treatment with Antibiotics.Antimicrob Agents Chemother. 2022 Jan 18;66(1):e0087821. doi: 10.1128/AAC.00878-21. Epub 2021 Nov 8. Antimicrob Agents Chemother. 2022. PMID: 34748386 Free PMC article.
References
-
- Horne JR, Erdmann VA. Isolation and characterization of 5S RNA-protein complexes from Bacillus stearothermophilus and Escherichia coli ribosomes. Mol Gen Genet. 1972;119:337–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

