Abnormal hair development and apparent follicular transformation to mammary gland in the absence of hedgehog signaling
- PMID: 17199044
- PMCID: PMC1885956
- DOI: 10.1016/j.devcel.2006.12.006
Abnormal hair development and apparent follicular transformation to mammary gland in the absence of hedgehog signaling
Abstract
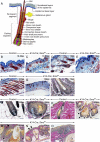
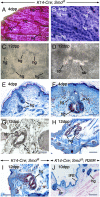
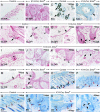
We show that removing the Shh signal tranducer Smoothened from skin epithelium secondarily results in excess Shh levels in the mesenchyme. Moreover, the phenotypes we observe reflect decreased epithelial Shh signaling, yet increased mesenchymal Shh signaling. For example, the latter contributes to exuberant hair follicle (HF) induction, while the former depletes the resulting follicular stem cell niches. This disruption of the niche apparently also allows the remaining stem cells to initiate hair formation at inappropriate times. Thus, the temporal structure of the hair cycle may depend on the physical structure of the niche. Finally, we find that the ablation of epithelial Shh signaling results in unexpected transformations: the follicular outer root sheath takes on an epidermal character, and certain HFs disappear altogether, having adopted a strikingly mammary gland-like fate. Overall, our study uncovers a multifaceted function for Shh in sculpting and maintaining the integrity and identity of the developing HF.
Figures




References
-
- Adolphe C., Narang M., Ellis T., Wicking C., Kaur P., Wainwright B.J. An in vivo comparative study of Sonic, Desert and Indian Hedgehog reveals that Hedgehog pathway activity regulates epidermal stem cell homeostasis. Development. 2004;131:5009–5019. - PubMed
-
- Chiang C., Swan R., Grachtchouk M., Bolinger M., Litingtung Y., Robertson E., Cooper M.K., Gaffield W., Westphal H., Beachy P., Dlugosz A.A. Essential role for sonic hedgehog during hair follicle morphogenesis. Dev. Biol. 1999;205:1–9. - PubMed
-
- Cohn B.A. In search of human skin pheromones. Arch. Dermatol. 1994;130:1048–1051. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

