Rapid turnover of mcl-1 couples translation to cell survival and apoptosis
- PMID: 17200126
- PMCID: PMC1831535
- DOI: 10.1074/jbc.M610643200
Rapid turnover of mcl-1 couples translation to cell survival and apoptosis
Abstract
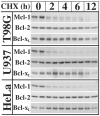
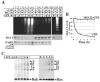
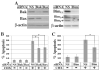
Inhibition of translation plays a role in apoptosis induced by a variety of stimuli, but the mechanism by which it promotes apoptosis has not been established. We have investigated the hypothesis that selective degradation of anti-apoptotic regulatory protein(s) is responsible for apoptosis resulting from translation inhibition. Induction of apoptosis by cycloheximide was detected within 2-4 h and blocked by proteasome inhibitors, indicating that degradation of short-lived protein(s) was required. Caspase inhibition and overexpression of Bcl-x(L) blocked cycloheximide-induced apoptosis. In addition, cycloheximide induced rapid activation of Bak and Bax, which required proteasome activity. Mcl-1 was degraded by the proteasome with a half-life of approximately 30 min following inhibition of protein synthesis, preceding Bak/Bax activation and the onset of apoptosis. Overexpression of Mcl-1 blocked apoptosis induced by cycloheximide, whereas RNA interference knockdown of Mcl-1 induced apoptosis. Knockdown of Bim and Bak, downstream targets of Mcl-1, inhibited cycloheximide-induced apoptosis, as did knockdown of Bax. Apoptosis resulting from inhibition of translation thus involves the rapid degradation of Mcl-1, leading to activation of Bim, Bak, and Bax. Because of its rapid turnover, Mcl-1 may serve as a convergence point for signals that affect global translation, coupling translation to cell survival and the apoptotic machinery.
Figures





Similar articles
-
Specific cleavage of Mcl-1 by caspase-3 in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in Jurkat leukemia T cells.J Biol Chem. 2005 Mar 18;280(11):10491-500. doi: 10.1074/jbc.M412819200. Epub 2005 Jan 6. J Biol Chem. 2005. PMID: 15637055
-
Cycloheximide Can Induce Bax/Bak Dependent Myeloid Cell Death Independently of Multiple BH3-Only Proteins.PLoS One. 2016 Nov 2;11(11):e0164003. doi: 10.1371/journal.pone.0164003. eCollection 2016. PLoS One. 2016. PMID: 27806040 Free PMC article.
-
Interrelated roles for Mcl-1 and BIM in regulation of TRAIL-mediated mitochondrial apoptosis.J Biol Chem. 2006 Apr 14;281(15):10153-63. doi: 10.1074/jbc.M510349200. Epub 2006 Feb 13. J Biol Chem. 2006. PMID: 16478725
-
Role of Bcl-2 family members in anoxia induced cell death.Cell Cycle. 2007 Apr 1;6(7):807-9. doi: 10.4161/cc.6.7.4044. Epub 2007 Apr 22. Cell Cycle. 2007. PMID: 17377500 Review.
-
Mcl-1: a highly regulated cell death and survival controller.J Biomed Sci. 2006 Mar;13(2):201-4. doi: 10.1007/s11373-005-9064-4. J Biomed Sci. 2006. PMID: 16456709 Review.
Cited by
-
The BCL-2 selective inhibitor ABT-199 sensitizes soft tissue sarcomas to proteasome inhibition by a concerted mechanism requiring BAX and NOXA.Cell Death Dis. 2020 Aug 24;11(8):701. doi: 10.1038/s41419-020-02910-2. Cell Death Dis. 2020. PMID: 32839432 Free PMC article.
-
Eliminating Legionella by inhibiting BCL-XL to induce macrophage apoptosis.Nat Microbiol. 2016 Feb 24;1:15034. doi: 10.1038/nmicrobiol.2015.34. Nat Microbiol. 2016. PMID: 27572165
-
Regulation of ubiquitination-mediated protein degradation by survival kinases in cancer.Front Oncol. 2012 Feb 20;2:15. doi: 10.3389/fonc.2012.00015. eCollection 2012. Front Oncol. 2012. PMID: 22649777 Free PMC article.
-
Phosphorylation of Mcl-1 by CDK1-cyclin B1 initiates its Cdc20-dependent destruction during mitotic arrest.EMBO J. 2010 Jul 21;29(14):2407-20. doi: 10.1038/emboj.2010.112. Epub 2010 Jun 4. EMBO J. 2010. PMID: 20526282 Free PMC article.
-
Hectd3 promotes pathogenic Th17 lineage through Stat3 activation and Malt1 signaling in neuroinflammation.Nat Commun. 2019 Feb 11;10(1):701. doi: 10.1038/s41467-019-08605-3. Nat Commun. 2019. PMID: 30741923 Free PMC article.
References
-
- Danial NN, Korsmeyer SJ. Cell. 2004;116:205–219. - PubMed
-
- Nakano K, Vousden KH. Mol. Cell. 2001;7:683–694. - PubMed
-
- Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. Mol. Cell. 2001;7:673–682. - PubMed
-
- Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N. Science. 2000;288:1053–1058. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

