Aggregated myocilin induces russell bodies and causes apoptosis: implications for the pathogenesis of myocilin-caused primary open-angle glaucoma
- PMID: 17200186
- PMCID: PMC1762699
- DOI: 10.2353/ajpath.2007.060806
Aggregated myocilin induces russell bodies and causes apoptosis: implications for the pathogenesis of myocilin-caused primary open-angle glaucoma
Abstract
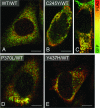
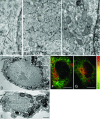
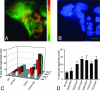
Primary open-angle glaucoma with elevated intraocular pressure is a leading cause of blindness worldwide. Mutations of myocilin are known to play a critical role in the manifestation of the disease. Misfolded mutant myocilin forms secretion-incompetent intracellular aggregates. The block of myocilin secretion was proposed to alter the extracellular matrix environment of the trabecular meshwork, with subsequent impediment of aqueous humor outflow leading to elevated intraocular pressure. However, the molecular pathogenesis of myocilin-caused glaucoma is poorly defined. In this study, we show that heteromeric complexes composed of wild-type and mutant myocilin were retained in the rough endoplasmic reticulum, aggregating to form inclusion bodies typical of Russell bodies. The presence of myocilin aggregates induced the unfolded protein response proteins BiP and phosphorylated endoplasmic reticulum-localized eukaryotic initiation factor-2alpha kinase (PERK) with the subsequent activation of caspases 12 and 3 and expression of C/EBP homologous protein (CHOP)/GADD153, leading to apoptosis. Our findings identify endoplasmic reticulum stress-induced apoptosis as a pathway to explain the reduction of trabecular meshwork cells in patients with myocilin-caused glaucoma. As a consequence, the phagocytotic capacity of the remaining trabecular meshwork cell population would be insufficient for effective cleaning of aqueous humor, constituting a major pathogenetic factor for the development of increased intraocular pressure in primary open-angle glaucoma.
Figures



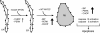
References
-
- Shields MB, Ritch R, Krupin T. Classification of the Glaucoma. Ritch R, Shields MB, Krupin T, editors. Philadelphia: Mosby,; 1996:pp 717–725.
-
- Tamm ER. Myocilin and glaucoma: facts and ideas. Prog Retin Eye Res. 2002;21:395–428. - PubMed
-
- Tan JC, Peters DM, Kaufman PL. Recent developments in understanding the pathophysiology of elevated intraocular pressure. Curr Opin Ophthalmol. 2006;17:168–174. - PubMed
-
- Grierson I, Hogg P. The proliferative and migratory activities of trabecular meshwork cells. Prog Retin Eye Res. 1995;15:33–67.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

