Unique matrix structure in the rough endoplasmic reticulum cisternae of pseudoachondroplasia chondrocytes
- PMID: 17200202
- PMCID: PMC1762700
- DOI: 10.2353/ajpath.2007.060530
Unique matrix structure in the rough endoplasmic reticulum cisternae of pseudoachondroplasia chondrocytes
Abstract
Mutations in cartilage oligomeric matrix protein (COMP) cause two skeletal dysplasias, pseudoachondroplasia (PSACH) and multiple epiphyseal dysplasia (MED/EDM1). Because COMP exists as a homopentamer, only one mutant COMP subunit may result in an abnormal complex that is accumulated in expanded rough endoplasmic reticulum (rER) cisternae, a hallmark of PSACH. Type IX collagen and matrilin-3 (MATN3), also accumulate in the rER cisternae of PSACH chondrocytes, but it is unknown how mutant COMP interacts with these proteins. The studies herein focus on defining the organization of these intracellularly retained proteins using fluorescence deconvolution microscopy. A unique matrix organization was identified in which type II procollagen formed a central core surrounded by a protein network of mutant COMP, type IX collagen, and MATN3. This pattern of matrix organization was found in multiple cisternae from single chondrocytes and in chondrocytes with different COMP mutations, indicating a common pattern of interaction. This suggests that stalling of mutant COMP and an interaction between mutant COMP and type II procollagen are initiating events in the assembly of matrix in the rER, possibly explaining why the material is not readily cleared from the rER. Altogether, these data suggest that mutant COMP initiates and perhaps catalyzes premature intracellular matrix assembly.
Figures
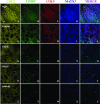


References
-
- Adams J, Tucker RP, Lawler J. New York: Springer-Verlag,; The Thrombospondin Gene Family. 1995
-
- DiCesare PE, Morgelin M, Mann K, Paulsson M. Cartilage oligomeric matrix protein and thrombospondin 1. Purification from articular cartilage, electron microscopic structure, and chondrocyte binding. Eur J Biochem. 1994;223:927–937. - PubMed
-
- Hecht JT, Deere M, Putnam E, Cole W, Vertel B, Chen H, Lawler J. Characterization of cartilage oligomeric matrix protein (COMP) in human normal and pseudoachondroplasia musculoskeletal tissues. Matrix Biol. 1998;17:269–278. - PubMed
-
- Hedbom E, Antonsson P, Hjerpe A, Aeschlimann D, Paulsson M, Rosa-Pimentel E, Sommarin Y, Wendel M, Oldberg A, Heinegard D. Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J Biol Chem. 1992;267:6132–6136. - PubMed
-
- Kato S, Yamada H, Terada N, Masuda K, Lenz ME, Morita M, Yoshihara Y, Henmi O. Joint biomarkers in idiopathic femoral head osteonecrosis: comparison with hip osteoarthritis. J Rheumatol. 2005;32:1518–1523. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

