Vasculoprotective effects of anti-tumor necrosis factor-alpha treatment in aging
- PMID: 17200210
- PMCID: PMC1762698
- DOI: 10.2353/ajpath.2007.060708
Vasculoprotective effects of anti-tumor necrosis factor-alpha treatment in aging
Abstract
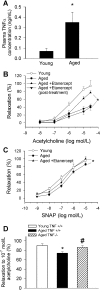
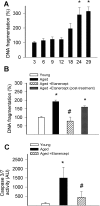
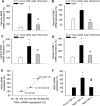
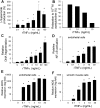
Vascular aging is associated with dysregulation of tumor necrosis factor (TNF)-alpha expression. TNF-alpha is a master regulator of vascular proatherogenic phenotypic changes, and it has been linked to endothelial dysfunction and apoptosis. To test the hypothesis that anti-TNF-alpha treatment exerts vasculoprotective effects in aging, aged (29 months old) F344 rats were treated with etanercept (1 mg/kg/week for 4 weeks), which binds and inactivates TNF-alpha. In aged carotid arteries, relaxations to acetylcholine were decreased, and endothelial O2* production was increased (as shown by dihydroethidine fluorescence measurements). Etanercept treatment significantly improved responses to acetylcholine and decreased vascular NAD(P)H oxidase activity and expression. In aged carotid and coronary arteries, there were increases in DNA fragmentation rate and caspase 3/7 activity (indicating an increased rate of apoptotic cell death), which were attenuated by etanercept treatment. In aged vessels, there was an up-regulation of inflammatory markers, including inducible nitric-oxide synthase and intercellular adhesion molecule-1, which was decreased by etanercept treatment. In carotid arteries of young animals, recombinant TNF-alpha elicited endothelial dysfunction, oxidative stress, and increased apoptosis and proinflammatory gene expression, mimicking many of the symptoms of vascular aging. Thus, we propose that anti-TNF-alpha treatment exerts anti-aging vasculoprotective effects.
Figures



References
-
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. - PubMed
-
- Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. - PubMed
-
- Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17:21–30. - PubMed
-
- Francia P, delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, Pelicci PG, Schiavoni M, Luscher TF, Volpe M, Cosentino F. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation. 2004;110:2889–2895. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

