Overexpression of Notch1 ectodomain in myeloid cells induces vascular malformations through a paracrine pathway
- PMID: 17200211
- PMCID: PMC1762695
- DOI: 10.2353/ajpath.2007.060351
Overexpression of Notch1 ectodomain in myeloid cells induces vascular malformations through a paracrine pathway
Abstract
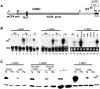
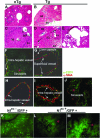
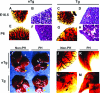
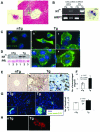
We previously reported that truncation of Notch1 (N1) by provirus insertion leads to overexpression of both the intracellular (N1(IC)) and the extracellular (N1(EC)) domains. We produced transgenic (Tg) mice expressing N1(EC) in T cells and in cells of the myeloid lineage under the regulation of the CD4 gene. These CD4C/N1(EC) Tg mice developed vascular disease, predominantly in the liver: superficial distorted vessels, cavernae, lower branching of parenchymal vessels, capillarized sinusoids, and aberrant smooth muscle/endothelial cell topography. The disease developed in lethally irradiated normal mice transplanted with Tg bone marrow or fetal liver cells as well as in Rag-/- Tg mice. In nude mice transplanted with fetal liver cells from (ROSA26 x CD4C/N1(EC)) F1 Tg mice, abnormal vessels were of recipient origin. Transplantation of Tg peritoneal macrophages into normal recipients also induced abnormal vessels. These Tg macrophages showed impaired functions, and their conditioned medium inhibited the proliferation of liver sinusoid endothelial cells in vitro. The Egr-1 gene and some of its targets (Jag1, FIII, FXIII-A, MCP-1, and MCP-5), previously implicated in hemangioma or vascular malformations, were overexpressed in Tg macrophages. These results show that myeloid cells can be reprogrammed by N1(EC) to induce vascular malformations through a paracrine pathway.
Figures



References
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
-
- Kopan R. Notch: a membrane-bound transcription factor. J Cell Sci. 2002;115:1095–1097. - PubMed
-
- Shawber CJ, Kitajewski J. Notch function in the vasculature: insights from zebrafish, mouse and man. Bioessays. 2004;26:225–234. - PubMed
-
- Rebay I, Fleming RJ, Fehon RG, Cherbas L, Cherbas P, Artavanis-Tsakonas S. Specific EGF repeats of Notch mediate interactions with Delta and Serrate: implications for Notch as a multifunctional receptor. Cell. 1991;67:687–699. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

