IL-25 regulates Th17 function in autoimmune inflammation
- PMID: 17200411
- PMCID: PMC2118427
- DOI: 10.1084/jem.20061738
IL-25 regulates Th17 function in autoimmune inflammation
Abstract
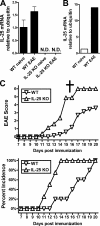
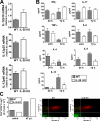
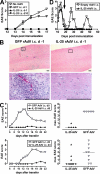
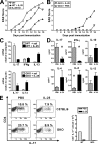
Interleukin (IL)-25 is a member of the IL-17 family of cytokines. However, unlike the other members of this family, IL-25 promotes T helper (Th) 2 responses. We now show that IL-25 also regulates the development of autoimmune inflammation mediated by IL-17-producing T cells. We have generated IL-25-deficient (il25-/-) mice and found that they are highly susceptible to experimental autoimmune encephalomyelitis (EAE). The accelerated disease in the il25-/- mice is associated with an increase of IL-23 in the periphery and a subsequent increase in the number of inflammatory IL-17-, IFNgamma-, and TNF-producing T cells that invade the central nervous system. Neutralization of IL-17 but not IFNgamma in il25-/- mice prevented EAE, suggesting that IL-17 is a major disease-promoting factor. IL-25 treatment at several time points during a relapse-remitting model or chronic model of EAE completely suppressed disease. IL-25 treatment induced elevated production of IL-13, which is required for suppression of Th17 responses by direct inhibition of IL-23, IL-1beta, and IL-6 expression in activated dendritic cells. Thus, IL-25 and IL-17, being members of the same cytokine family, play opposing roles in the pathogenesis of organ-specific autoimmunity.
Figures

References
-
- Fort, M.M., J. Cheung, D. Yen, J. Li, S.M. Zurawski, S. Lo, S. Menon, T. Clifford, B. Hunte, R. Lesley, et al. 2001. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 15:985–995. - PubMed
-
- Hurst, S.D., T. Muchamuel, D.M. Gorman, J.M. Gilbert, T. Clifford, S. Kwan, S. Menon, B. Seymour, C. Jackson, T.T. Kung, et al. 2002. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J. Immunol. 169:443–453. - PubMed
-
- Ikeda, K., H. Nakajima, K. Suzuki, S. Kagami, K. Hirose, A. Suto, Y. Saito, and I. Iwamoto. 2003. Mast cells produce interleukin-25 upon Fc epsilon RI-mediated activation. Blood. 101:3594–3596. - PubMed
-
- Kang, C.M., A.S. Jang, M.H. Ahn, J.A. Shin, J.H. Kim, Y.S. Choi, T.Y. Rhim, and C.S. Park. 2005. Interleukin-25 and interleukin-13 production by alveolar macrophages in response to particles. Am. J. Respir. Cell Mol. Biol. 33:290–296. - PubMed
-
- Owyang, A.M., C. Zaph, E.H. Wilson, K.J. Guild, T. McClanahan, H.R. Miller, D.J. Cua, M. Goldschmidt, C.A. Hunter, R.A. Kastelein, and D. Artis. 2006. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J. Exp. Med. 203:843–849. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

