Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease
- PMID: 17200412
- PMCID: PMC2118432
- DOI: 10.1084/jem.20061852
Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease
Abstract
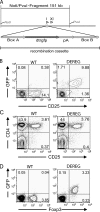
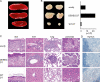
The scurfy mutant mouse strain suffers from a fatal lymphoproliferative disease leading to early death within 3-4 wk of age. A frame-shift mutation of the forkhead box transcription factor Foxp3 has been identified as the molecular cause of this multiorgan autoimmune disease. Foxp3 is a central control element in the development and function of regulatory T cells (T reg cells), which are necessary for the maintenance of self-tolerance. However, it is unclear whether dysfunction or a lack of T reg cells is etiologically involved in scurfy pathogenesis and its human correlate, the IPEX syndrome. We describe the generation of bacterial artificial chromosome-transgenic mice termed "depletion of regulatory T cell" (DEREG) mice expressing a diphtheria toxin (DT) receptor-enhanced green fluorescent protein fusion protein under the control of the foxp3 gene locus, allowing selective and efficient depletion of Foxp3+ T reg cells by DT injection. Ablation of Foxp3+ T reg cells in newborn DEREG mice led to the development of scurfy-like symptoms with splenomegaly, lymphadenopathy, insulitis, and severe skin inflammation. Thus, these data provide experimental evidence that the absence of Foxp3+ T reg cells is indeed sufficient to induce a scurfy-like phenotype. Furthermore, DEREG mice will allow a more precise definition of the function of Foxp3+ T reg cells in immune reactions in vivo.
Figures


References
-
- Sakaguchi, S. 2005. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 6:345–352. - PubMed
-
- von Boehmer, H. 2005. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 6:338–344. - PubMed
-
- Coombes, J.L., N.J. Robinson, K.J. Maloy, H.H. Uhlig, and F. Powrie. 2005. Regulatory T cells and intestinal homeostasis. Immunol. Rev. 204:184–194. - PubMed
-
- Taguchi, O., and T. Takahashi. 1996. Administration of anti-interleukin-2 receptor alpha antibody in vivo induces localized autoimmune disease. Eur. J. Immunol. 26:1608–1612. - PubMed
-
- McHugh, R.S., and E.M. Shevach. 2002. Cutting edge: depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J. Immunol. 168:5979–5983. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

