Two distinct modes of myosin assembly and dynamics during epithelial wound closure
- PMID: 17200415
- PMCID: PMC2063619
- DOI: 10.1083/jcb.200609116
Two distinct modes of myosin assembly and dynamics during epithelial wound closure
Erratum in
- J Cell Biol. 2007 Feb 12;176(4):545
Abstract
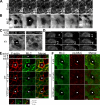
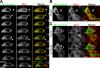
Actomyosin contraction powers the sealing of epithelial sheets during embryogenesis and wound closure; however, the mechanisms are poorly understood. After laser ablation wounding of Madin-Darby canine kidney cell monolayers, we observed distinct steps in wound closure from time-lapse images of myosin distribution during resealing. Immediately upon wounding, actin and myosin II regulatory light chain accumulated at two locations: (1) in a ring adjacent to the tight junction that circumscribed the wound and (2) in fibers at the base of the cell in membranes extending over the wound site. Rho-kinase activity was required for assembly of the myosin ring, and myosin II activity was required for contraction but not for basal membrane extension. As it contracted, the myosin ring moved toward the basal membrane with ZO-1 and Rho-kinase. Thus, we suggest that tight junctions serve as attachment points for the actomyosin ring during wound closure and that Rho-kinase is required for localization and activation of the contractile ring.
Figures



References
-
- Bertet, C., L. Sulak, and T. Lecuit. 2004. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 429:667–671. - PubMed
-
- Ikebe, M., and D.J. Hartshorne. 1985. Phosphorylation of smooth muscle myosin at two distinct sites by myosin light chain kinase. J. Biol. Chem. 260:10027–10031. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

