Interphotoreceptor retinoid-binding protein gene structure in tetrapods and teleost fish
- PMID: 17200656
- PMCID: PMC2898515
Interphotoreceptor retinoid-binding protein gene structure in tetrapods and teleost fish
Abstract
Purpose: The interphotoreceptor retinoid-binding protein (IRBP) gene possesses an unusual structure, encoding multiple Repeats, each consisting of about 300 amino acids. Our goals were to gain insight into the function of IRBP, and to test the current model for the evolution of IRBP, in which Repeats were replicated from a simpler ancestral gene.
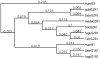
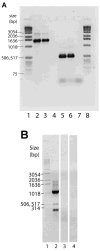
Methods: We employed a bioinformatics approach to analyze IRBP loci in recently completed or near-complete genome sequences of several vertebrates and nonvertebrate chordates. IRBP gene expression in zebrafish was evaluated by reverse transcriptase PCR (RT-PCR) and in situ mRNA hybridizations with gene-specific probes.
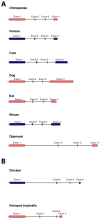
Results: Patterns of exons and introns in the IRBP genes of tetrapods were highly similar, as were predicted amino acid sequences and Repeat structures. IRBP gene structure in teleost fish was more variable, and we report a new gene structure for two species, the Japanese puffer fish (Takifugu rubripes) and the zebrafish (Danio rerio). These teleost genomes contain a two-gene IRBP locus arranged head-to-tail in which the first gene, Gene 1, is intronless and contains a single large exon encoding three complete Repeats. It is followed by a second gene, Gene 2, which corresponds to the previously reported gene consisting of two Repeats spread across four exons and three introns. Each of the two zebrafish genes is transcribed. Gene 2 is expressed in the photoreceptors and RPE, and Gene 1 is expressed in the inner nuclear layer and weakly in the ganglion cell layer.
Conclusions: The tetrapod IRBP gene structure is highly conserved while the teleost fish gene structure was a surprise: It appears to be a two-gene locus with distinct Repeat organization in each open reading frame. This gene structure and gene expression data are consistent with possible neofunctionalization or sub-function partitioning of Gene 1 and Gene 2 in the zebrafish. We suggest that the two-gene locus in teleost fish arose as a consequence of either the known whole genome duplication or single gene tandem duplication.
Figures








References
-
- Crouch RK, Hazard ES, Lind T, Wiggert B, Chader G, Corson DW. Interphotoreceptor retinoid-binding protein and alpha-tocopherol preserve the isomeric and oxidation state of retinol. Photochem Photobiol. 1992;56:251–5. - PubMed
-
- Gonzalez-Fernandez F. Evolution of the visual cycle: the role of retinoid-binding proteins. J Endocrinol. 2002;175:75–88. - PubMed
-
- Liou GI, Wang M, Matragoon S. Timing of interphotoreceptor retinoid-binding protein (IRBP) gene expression and hypomethylation in developing mouse retina. Dev Biol. 1994;161:345–56. - PubMed
-
- Gonzalez-Fernandez F, Van Niel E, Edmonds C, Beaver H, Nickerson JM, Garcia-Fernandez JM, Campohiaro PA, Foster RG. Differential expression of interphotoreceptor retinoid-binding protein, opsin, cellular retinaldehyde-binding protein, and basic fibroblastic growth factor. Exp Eye Res. 1993;56:411–27. Erratum in: Exp Eye Res 1993 Jul;57(1):127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY016470/EY/NEI NIH HHS/United States
- R01EY012146/EY/NEI NIH HHS/United States
- R24EY017045/EY/NEI NIH HHS/United States
- K12 GM000680/GM/NIGMS NIH HHS/United States
- R01EY016470/EY/NEI NIH HHS/United States
- R24 EY017045/EY/NEI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01 EY012146/EY/NEI NIH HHS/United States
- K12GM000680/GM/NIGMS NIH HHS/United States
- R03 EY013986/EY/NEI NIH HHS/United States
- P30 EY006360/EY/NEI NIH HHS/United States
- P30EY006360/EY/NEI NIH HHS/United States
- R03EY013986/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

