Ménétrier disease and gastrointestinal stromal tumors: hyperproliferative disorders of the stomach
- PMID: 17200708
- PMCID: PMC1716220
- DOI: 10.1172/JCI30491
Ménétrier disease and gastrointestinal stromal tumors: hyperproliferative disorders of the stomach
Abstract
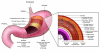
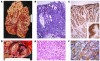
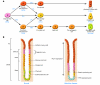
Ménétrier disease and gastrointestinal stromal tumors (GISTs) are hyperproliferative disorders of the stomach caused by dysregulated receptor tyrosine kinases (RTKs). In Ménétrier disease, overexpression of TGF-alpha, a ligand for the RTK EGFR, results in selective expansion of surface mucous cells in the body and fundus of the stomach. In GISTs, somatic mutations of the genes encoding the RTK KIT (or PDGFRA in a minority of cases) result in constitutive kinase activity and neoplastic transformation of gut pacemaker cells (interstitial cells of Cajal). On the basis of the involvement of these RTKs in the pathogenesis of these disorders, Ménétrier disease patients have been effectively treated with a blocking monoclonal antibody specific for EGFR and GIST patients with KIT and PDGFRA tyrosine kinase inhibitors.
Figures
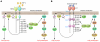
References
-
- Ménétrier P. Des polyadenomes gastriques et leur rapport avec le cancer de l’estomac. Arch. Physiol. Norm. Pathol. 1888;1:32–55; 236–262.
-
- Occena R.O., Taylor S.F., Robinson C.C., Sokol R.J. Association of cytomegalovirus with Ménétrier’s disease in childhood: report of two new cases with a review of literature. J. Pediatr. Gastroenterol. Nutr. 1993;17:217–224. - PubMed
-
- Scharschmidt B.F. The natural history of hypertrophic gastrophy (Ménétrier’s disease). Report of a case with 16 year follow-up and review of 120 cases from the literature. Am. J. Med. 1977;63:644–652. - PubMed
-
- Vandenborre K.M., et al. Hypertrophic lymphocytic gastritis with a gastric carcinoma. Eur. J. Gastroenterol. Hepatol. 1998;10:797–801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

