Beta blocker specificity: a building block toward personalized medicine
- PMID: 17200711
- PMCID: PMC1716219
- DOI: 10.1172/JCI30476
Beta blocker specificity: a building block toward personalized medicine
Abstract
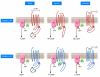
Drugs known as beta blockers, which antagonize the beta-adrenergic receptor (beta-AR), are an important component of the treatment regimen for chronic heart failure (HF). However, a significant body of evidence indicates that genetic heterogeneity at the level of the beta(1)-AR may be a factor in explaining the variable responses of HF patients to beta blockade. In this issue of the JCI, Rochais et al. describe how a single amino acid change in beta(1)-AR alters its structural conformation and improves its functional response to carvedilol, a beta blocker currently used in the treatment of HF (see the related article beginning on page 229). This may explain why some HF patients have better responses not only to carvedilol but to certain other beta blockers as well. The data greatly enhance our mechanistic understanding of myocardial adrenergic signaling and support the development of "tailored" or "personalized" medicine, in which specific therapies could be prescribed based on a patient's genotype.
Figures
Comment on
- J Clin Invest. 117:229.
Similar articles
-
Genetic tailoring of pharmacotherapy in heart failure: optimize the old, while we wait for something new.J Card Fail. 2012 Apr;18(4):338-49. doi: 10.1016/j.cardfail.2012.01.002. Epub 2012 Feb 2. J Card Fail. 2012. PMID: 22464776 Review.
-
Carvedilol use at discharge in patients hospitalized for heart failure is associated with improved survival: an analysis from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF).Am Heart J. 2007 Jan;153(1):82.e1-11. doi: 10.1016/j.ahj.2006.10.008. Am Heart J. 2007. PMID: 17174643
-
Lack of association between adrenergic receptor genotypes and survival in heart failure patients treated with carvedilol or metoprolol.J Am Coll Cardiol. 2008 Aug 19;52(8):644-51. doi: 10.1016/j.jacc.2008.05.022. Epub 2008 Jun 23. J Am Coll Cardiol. 2008. PMID: 18702968
-
Impact of beta1-adrenergic receptor polymorphisms on susceptibility to heart failure, arrhythmogenesis, prognosis, and response to beta-blocker therapy.Am J Cardiol. 2008 Sep 15;102(6):726-32. doi: 10.1016/j.amjcard.2008.04.070. Epub 2008 Jul 25. Am J Cardiol. 2008. PMID: 18773997
-
Beta-blocker pharmacogenetics in heart failure.Heart Fail Rev. 2010 May;15(3):187-96. doi: 10.1007/s10741-008-9094-x. Epub 2008 Apr 24. Heart Fail Rev. 2010. PMID: 18437562 Free PMC article. Review.
Cited by
-
Radionuclide imaging of neurohormonal system of the heart.Theranostics. 2015 Feb 15;5(6):545-58. doi: 10.7150/thno.10900. eCollection 2015. Theranostics. 2015. PMID: 25825596 Free PMC article. Review.
-
Pharmacogenomics in cardiovascular disorders: Steps in approaching personalized medicine in cardiovascular medicine.Pharmgenomics Pers Med. 2009;2:59-67. doi: 10.2147/pgpm.s5805. Epub 2009 Sep 8. Pharmgenomics Pers Med. 2009. PMID: 23226035 Free PMC article.
-
Crystal structures of a stabilized β1-adrenoceptor bound to the biased agonists bucindolol and carvedilol.Structure. 2012 May 9;20(5):841-9. doi: 10.1016/j.str.2012.03.014. Structure. 2012. PMID: 22579251 Free PMC article.
-
Pro-survival function of MEF2 in cardiomyocytes is enhanced by β-blockers.Cell Death Discov. 2015 Sep 14;1:15019. doi: 10.1038/cddiscovery.2015.19. eCollection 2015. Cell Death Discov. 2015. PMID: 27551452 Free PMC article.
-
Association of Ovarian Tumor β2-Adrenergic Receptor Status with Ovarian Cancer Risk Factors and Survival.Cancer Epidemiol Biomarkers Prev. 2016 Dec;25(12):1587-1594. doi: 10.1158/1055-9965.EPI-16-0534. Epub 2016 Sep 1. Cancer Epidemiol Biomarkers Prev. 2016. PMID: 27587791 Free PMC article.
References
-
- Lee T.H.2001Management of heart failure: AHA/ACC guidelines summary. Heart disease: a textbook of cardiovascular medicine. 6th editio n . E. Braunwald, editor. 101652–685.
-
- Rockman H.A., Koch W.J., Lefkowitz R.J. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. - PubMed
-
- Maqbool A., Hall S.A., Ball S.G., Balmforth A.J. Common polymorphisms of β1–adrenoreceptor: identification and rapid screening assay [research letter]. . Lancet. 1999;353:897. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

