Atherogenic remnant lipoproteins: role for proteoglycans in trapping, transferring, and internalizing
- PMID: 17200713
- PMCID: PMC1716223
- DOI: 10.1172/JCI30889
Atherogenic remnant lipoproteins: role for proteoglycans in trapping, transferring, and internalizing
Abstract
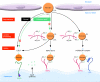
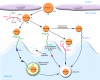
Unraveling the mechanisms controlling remnant lipoprotein clearance is important, as these lipoproteins are highly atherogenic. The most critical molecule in this process is apoE, which mediates high-affinity binding of remnant lipoproteins to members of the LDL receptor (LDLR) family and cell-surface heparan sulfate proteoglycans (HSPGs), which have been shown to play major independent as well as cooperative roles in remnant lipoprotein clearance. While all the players may have been identified, our understanding of how they interact and function together continues to evolve. In this issue of the JCI, MacArthur et al. (see the related article beginning on page 153) demonstrated that HSPGs under normal physiological conditions are critically important in the clearance of remnant lipoproteins, independent of LDLR family members. The complexity of VLDL and chylomicron remnant clearance was exemplified by the studies of Jones et al., also in this issue (see the related article beginning on page 165). Despite defective clearance of LDL in mice with a deficiency in the adaptor protein controlling internalization of the LDLR, called autosomal recessive hypercholesterolemia (ARH), remnant lipoprotein clearance was not grossly abnormal. A likely explanation is that the abnormal LDLRs bind the remnants and then transfer them to another acceptor for internalization. While the studies clearly demonstrate that the LDLR-related protein 1 is not involved and suggest a role for an additional unidentified receptor, it remains a possibility that HSPGs are responsible for remnant uptake by hepatocytes in the presence of defective LDLR internalization.
Figures
Comment on
-
Liver heparan sulfate proteoglycans mediate clearance of triglyceride-rich lipoproteins independently of LDL receptor family members.J Clin Invest. 2007 Jan;117(1):153-64. doi: 10.1172/JCI29154. J Clin Invest. 2007. PMID: 17200715 Free PMC article.
-
Disruption of LDL but not VLDL clearance in autosomal recessive hypercholesterolemia.J Clin Invest. 2007 Jan;117(1):165-74. doi: 10.1172/JCI29415. J Clin Invest. 2007. PMID: 17200716 Free PMC article.
References
-
- Mahley R.W., Ji Z.-S. Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J. Lipid Res. . 1999;40:1–16. - PubMed
-
- Mahley R.W., Huang Y., Rall S.C. Pathogenesis of type III hyperlipoproteinemia (dysbetalipoproteinemia): questions, quandaries, and paradoxes. J. Lipid Res. . 1999;40:1933–1949. - PubMed
-
- Mahley R.W., Weisgraber K.H., Innerarity T.L., Rall S.C. Genetic defects in lipoprotein metabolism. Elevation of atherogenic lipoproteins caused by impaired catabolism. JAMA. . 1991;265:78–83. - PubMed
-
- Mahley, R.W., and Rall, S.C., Jr. 1995. Type III hyperlipoproteinemia (dysbetalipoproteinemia): the role of apolipoprotein E in normal and abnormal lipoprotein metabolism. InThe metabolic and molecular bases of inherited disease. 7th edition. Volume 2. C.R. Scriver, A.L. Beaudet, W.S. Sly, and D. Valle, editors. McGraw-Hill. New York, New York, USA. 1953–1980.
-
- Mahley R.W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. . Science. . 1988;240:622–630. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

