The death domain superfamily in intracellular signaling of apoptosis and inflammation
- PMID: 17201679
- PMCID: PMC2904440
- DOI: 10.1146/annurev.immunol.25.022106.141656
The death domain superfamily in intracellular signaling of apoptosis and inflammation
Abstract
The death domain (DD) superfamily comprising the death domain (DD) subfamily, the death effector domain (DED) subfamily, the caspase recruitment domain (CARD) subfamily, and the pyrin domain (PYD) subfamily is one of the largest domain superfamilies. By mediating homotypic interactions within each domain subfamily, these proteins play important roles in the assembly and activation of apoptotic and inflammatory complexes. In this chapter, we review the molecular complexes assembled by these proteins, the structural and biochemical features of these domains, and the molecular interactions mediated by them. By analyzing the potential molecular basis for the function of these domains, we hope to provide a comprehensive understanding of the function, structure, interaction, and evolution of this important family of domains.
Figures


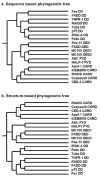
References
-
- Duvall E, Wyllie AH. Death and the cell. Immunol today. 1986;7:115–19. - PubMed
-
- Rathmell JC, Thompson CB. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell. 2002;109(Suppl):S97–107. - PubMed
-
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–61. - PubMed
-
- Salvesen GS. Caspases and apoptosis. Essays Biochem. 2002;38:9–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

