Protocol for the smoking, nicotine and pregnancy (SNAP) trial: double-blind, placebo-randomised, controlled trial of nicotine replacement therapy in pregnancy
- PMID: 17201904
- PMCID: PMC1764871
- DOI: 10.1186/1472-6963-7-2
Protocol for the smoking, nicotine and pregnancy (SNAP) trial: double-blind, placebo-randomised, controlled trial of nicotine replacement therapy in pregnancy
Abstract
Background: Smoking in pregnancy remains a public health challenge. Nicotine replacement therapy (NRT) is effective for smoking cessation in non-pregnant people, but because women metabolise nicotine and cotinine much faster in pregnancy, it is unclear whether this will be effective for smoking cessation in pregnancy. The NHS Health Technology Assessment Programme (HTA)-funded smoking, nicotine and pregnancy (SNAP) trial will investigate whether or not nicotine replacement therapy (NRT) is effective, cost-effective and safe when used for smoking cessation by pregnant women.
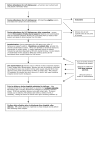
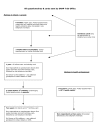
Methods/design: Over two years, in 5 trial centres, 1050 pregnant women who are between 12 and 24 weeks pregnant will be randomised as they attend hospital for ante-natal ultrasound scans. Women will receive either nicotine or placebo transdermal patches with behavioural support. The primary outcome measure is biochemically-validated, self-reported, prolonged and total abstinence from smoking between a quit date (defined before randomisation and set within two weeks of this) and delivery. At six months after childbirth self-reported maternal smoking status will be ascertained and two years after childbirth, self-reported maternal smoking status and the behaviour, cognitive development and respiratory symptoms of children born in the trial will be compared in both groups.
Discussion: This trial is designed to ascertain whether or not standard doses of NRT (as transdermal patches) are effective and safe when used for smoking cessation during pregnancy.
Figures
References
-
- Owen L, Penn G. Smoking and pregnancy: A survey of knowledge attitudes and behaviour, 1992–1999. London, Health Development Agency; 1999.
-
- Charlton A. Children and smoking: the family circle. British Medical Bulletin. 1996;52:90–107. - PubMed
-
- Silagy C. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews. 2000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

