Cytotoxic T-lymphocyte responses to human papillomavirus type 16 E5 and E7 proteins and HLA-A*0201-restricted T-cell peptides in cervical cancer patients
- PMID: 17202211
- PMCID: PMC1865983
- DOI: 10.1128/JVI.02256-06
Cytotoxic T-lymphocyte responses to human papillomavirus type 16 E5 and E7 proteins and HLA-A*0201-restricted T-cell peptides in cervical cancer patients
Abstract
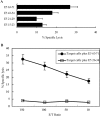
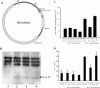
Previously, we found that human papillomavirus type 16 (HPV-16) E5 protein is a tumor rejection antigen and can induce cytotoxic T-lymphocyte (CTL) activity. Therefore, in this study, human leukocyte antigen A*0201 (HLA-A*0201)-restricted human CTL epitopes of HPV-16 E5 protein were identified using a bioinformatics approach, and the abilities of these predicted peptides to induce an immune response in HLA-A*0201 transgenic mice were confirmed by assaying E5-specific CTLs and in vitro-generated CTLs from normal peripheral blood T lymphocytes of HLA-A2-positive human donors. Second, the CTL responses to HLA-A*0201 CTL epitopes (E5 63-71 and E7 11-20) were examined in HPV-16-infected patients with HLA-A2. Third, the effect of HLA-A-type alleles on CTL activities in response to the entire E5 and E7 proteins was examined in cervical cancer patients. E5 and E7 peptides (but not the whole proteins) stimulated E5- and E7-specific CTL recall responses in HPV-16- and HLA-A2-positive cervical cancer patients, and HPV-16 E5 and E7 proteins stimulated naïve T cells in HPV-16-negative cervical cancer patients with HLA-A11 and -A24 haplotypes. In summary, this is the first demonstration that E5 63-71 is an HLA-A*0201-restricted T-cell epitope of HPV-16 E5.
Figures



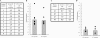

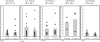
Similar articles
-
Identification of an HLA-A24-restricted cytotoxic T lymphocyte epitope from human papillomavirus type-16 E6: the combined effects of bortezomib and interferon-gamma on the presentation of a cryptic epitope.Int J Cancer. 2007 Feb 1;120(3):594-604. doi: 10.1002/ijc.22312. Int J Cancer. 2007. PMID: 17096336
-
Use of fluorogenic histocompatibility leukocyte antigen-A*0201/HPV 16 E7 peptide complexes to isolate rare human cytotoxic T-lymphocyte-recognizing endogenous human papillomavirus antigens.Cancer Res. 2000 Jan 15;60(2):365-71. Cancer Res. 2000. PMID: 10667589
-
Antitumor activity of human papillomavirus type 16 E7-specific T cells against virally infected squamous cell carcinoma of the head and neck.Cancer Res. 2005 Dec 1;65(23):11146-55. doi: 10.1158/0008-5472.CAN-05-0772. Cancer Res. 2005. PMID: 16322265
-
Human papillomavirus type 16 E5 protein as a therapeutic target.Yonsei Med J. 2006 Feb 28;47(1):1-14. doi: 10.3349/ymj.2006.47.1.1. Yonsei Med J. 2006. Retraction in: Yonsei Med J. 2011 May;52(3):551. doi: 10.3349/ymj.2011.52.3.551. PMID: 16502480 Free PMC article. Retracted. Review.
-
Immune responses against human papillomavirus (HPV) infection and evasion of host defense in cervical cancer.J Infect Chemother. 2012 Dec;18(6):807-15. doi: 10.1007/s10156-012-0485-5. Epub 2012 Nov 3. J Infect Chemother. 2012. PMID: 23117294 Review.
Cited by
-
Cytolytic activity of the human papillomavirus type 16 E711-20 epitope-specific cytotoxic T lymphocyte is enhanced by heat shock protein 110 in HLA-A*0201 transgenic mice.Clin Vaccine Immunol. 2013 Jul;20(7):1027-33. doi: 10.1128/CVI.00721-12. Epub 2013 May 8. Clin Vaccine Immunol. 2013. PMID: 23658393 Free PMC article.
-
A comprehensive in silico analysis for identification of therapeutic epitopes in HPV16, 18, 31 and 45 oncoproteins.PLoS One. 2018 Oct 24;13(10):e0205933. doi: 10.1371/journal.pone.0205933. eCollection 2018. PLoS One. 2018. PMID: 30356257 Free PMC article.
-
Differences in Extracellular Vesicle Protein Cargo Are Dependent on Head and Neck Squamous Cell Carcinoma Cell of Origin and Human Papillomavirus Status.Cancers (Basel). 2021 Jul 23;13(15):3714. doi: 10.3390/cancers13153714. Cancers (Basel). 2021. PMID: 34359613 Free PMC article.
-
HPV 16 E5 oncoprotein is expressed in early stage carcinogenesis and can be a target of immunotherapy.Hum Vaccin Immunother. 2017 Feb;13(2):291-297. doi: 10.1080/21645515.2017.1264777. Epub 2016 Dec 8. Hum Vaccin Immunother. 2017. PMID: 27929754 Free PMC article.
-
hrHPV E5 oncoprotein: immune evasion and related immunotherapies.J Exp Clin Cancer Res. 2017 May 25;36(1):71. doi: 10.1186/s13046-017-0541-1. J Exp Clin Cancer Res. 2017. PMID: 28545552 Free PMC article. Review.
References
-
- Ashrafi, G. H., M. R. Haghshenas, B. Marchetti, P. M. O'Brien, and M. S. Campo. 2005. E5 protein of human papillomavirus type 16 selectively downregulates surface HLA class I. Int. J. Cancer 113:276-283. - PubMed
-
- Bontkes, H. J., J. M. Walboomers, C. J. Meijer, T. J. Helmerhorst, and P. L. Stern. 1998. Specific MHC class I down-regulation is an early event in cervical dysplasia associated with clinical progression. Lancet 351:187-188. - PubMed
-
- Bontkes, H. J., T. D. de Gruijl, A. J. van den Muysenberg, R. H. Verheijen, M. J. Stukart, C. J. Meijer, R. J. Scheper, S. N. Stacey, M. F. Duggan-Keen, P. L. Stern, S. Man, L. K. Borysiewicz, and J. M. Walboomers. 2000. Human papillomavirus type 16 E6/E7-specific cytotoxic T lymphocytes in women with cervical neoplasia. Int. J. Cancer 88:92-98. - PubMed
-
- Bontkes, H. J., T. D. de Gruijl, J. M. Walboomers, A. J. van den Muysenberg, A. W. Gunther, R. J. Scheper, C. J. Meijer, and J. A. Kummer. 1997. Assessment of cytotoxic T-lymphocyte phenotype using the specific markers granzyme B and TIA-1 in cervical neoplastic lesions. Br. J. Cancer 76:1353-1360. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

