Initiation at the third in-frame AUG codon of open reading frame 3 of the hepatitis E virus is essential for viral infectivity in vivo
- PMID: 17202216
- PMCID: PMC1866010
- DOI: 10.1128/JVI.02259-06
Initiation at the third in-frame AUG codon of open reading frame 3 of the hepatitis E virus is essential for viral infectivity in vivo
Abstract
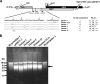
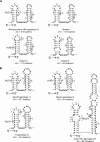
To determine the initiation strategy of the hepatitis E virus (HEV) open reading frame 3 (ORF3), we constructed five HEV mutants with desired mutations in the ORF1 and ORF2 junction region and tested their levels of in vivo infectivity in pigs. A mutant with a C-terminally truncated ORF3 is noninfectious in pigs, indicating that an intact ORF3 is required for in vivo infectivity. Mutations with substitutions in the first in-frame AUG in the junction region or with the same T insertion at the corresponding position of HEV genotype 4 did not affect the virus infectivity or rescue, although mutations with combinations of the two affected virus recovery efficiency, and a single mutation at the third in-frame AUG completely abolished virus infectivity in vivo, indicating that the third in-frame AUG in the junction region is required for virus infection and is likely the authentic initiation site for ORF3. A conserved double stem-loop RNA structure, which may be important for HEV replication, was identified in the junction region. This represents the first report of using a unique homologous pig model system to study the molecular mechanism of HEV replication and to systematically and definitively identify the authentic ORF3 initiation site.
Figures


Similar articles
-
Roles of the genomic sequence surrounding the stem-loop structure in the junction region including the 3' terminus of open reading frame 1 in hepatitis E virus replication.J Med Virol. 2018 Sep;90(9):1524-1531. doi: 10.1002/jmv.25215. Epub 2018 May 25. J Med Virol. 2018. PMID: 29718575 Free PMC article.
-
The open reading frame 3 gene of hepatitis E virus contains a cis-reactive element and encodes a protein required for infection of macaques.J Virol. 2005 Jun;79(11):6680-9. doi: 10.1128/JVI.79.11.6680-6689.2005. J Virol. 2005. PMID: 15890906 Free PMC article.
-
Primary structure of open reading frame 2 and 3 of the hepatitis E virus isolated from Morocco.J Med Virol. 1999 Feb;57(2):126-33. J Med Virol. 1999. PMID: 9892396
-
Hepatitis E Virus Replication.Viruses. 2019 Aug 6;11(8):719. doi: 10.3390/v11080719. Viruses. 2019. PMID: 31390784 Free PMC article. Review.
-
Recent advances in Hepatitis E virus.J Viral Hepat. 2010 Mar;17(3):153-61. doi: 10.1111/j.1365-2893.2009.01257.x. Epub 2009 Dec 21. J Viral Hepat. 2010. PMID: 20040046 Review.
Cited by
-
Hepatitis E Virus Genotype 3 Genomes from RNA-Positive but Serologically Negative Plasma Donors Have CUG as the Start Codon for ORF3.Intervirology. 2018;61(2):96-103. doi: 10.1159/000491926. Epub 2018 Oct 2. Intervirology. 2018. PMID: 30278453 Free PMC article.
-
The ORF3 protein of hepatitis E virus delays degradation of activated growth factor receptors by interacting with CIN85 and blocking formation of the Cbl-CIN85 complex.J Virol. 2010 Apr;84(8):3857-67. doi: 10.1128/JVI.01994-09. Epub 2010 Feb 3. J Virol. 2010. PMID: 20130058 Free PMC article.
-
Hepatitis e: molecular virology and pathogenesis.J Clin Exp Hepatol. 2013 Jun;3(2):114-24. doi: 10.1016/j.jceh.2013.05.001. Epub 2013 May 30. J Clin Exp Hepatol. 2013. PMID: 25755485 Free PMC article. Review.
-
Avian Hepatitis E Virus: With the Trend of Genotypes and Host Expansion.Front Microbiol. 2019 Jul 24;10:1696. doi: 10.3389/fmicb.2019.01696. eCollection 2019. Front Microbiol. 2019. PMID: 31396195 Free PMC article. Review.
-
Origin, antigenicity, and function of a secreted form of ORF2 in hepatitis E virus infection.Proc Natl Acad Sci U S A. 2018 May 1;115(18):4773-4778. doi: 10.1073/pnas.1721345115. Epub 2018 Apr 18. Proc Natl Acad Sci U S A. 2018. PMID: 29669922 Free PMC article.
References
-
- Emerson, S. U., D. Anderson, A. Arankalle, X. J. Meng, M. Purdy, G. G. Schlauder, and S. A. Tsarev. 2004. Hepevirus, p. 851-855. In C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy. VIIIth report of the ICTV. Elsevier/Academic Press, London, United Kingdom.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

