The IE2 60-kilodalton and 40-kilodalton proteins are dispensable for human cytomegalovirus replication but are required for efficient delayed early and late gene expression and production of infectious virus
- PMID: 17202222
- PMCID: PMC1865986
- DOI: 10.1128/JVI.02454-06
The IE2 60-kilodalton and 40-kilodalton proteins are dispensable for human cytomegalovirus replication but are required for efficient delayed early and late gene expression and production of infectious virus
Abstract
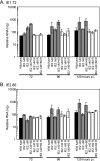
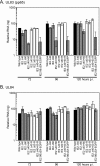
The human cytomegalovirus (HCMV) IE2 86-kDa protein is an essential transactivator of viral and cellular gene expression. Additional proteins of 60 and 40 kDa are expressed from the IE2 gene at late times postinfection and are identical to the C terminus of IE2 86. We have constructed HCMV recombinants that express wild-type full-length IE2 86 but do not express the IE2 40- and 60-kDa proteins. Each of these recombinants is viable, indicating that neither the 60-kDa nor the 40-kDa protein is required for virus replication, either alone or in combination. Cells infected with the IE2 60 and IE2 40 deletion mutants, however, exhibit decreased expression of selected viral genes at late times. In particular, expression of the viral DNA replication factor UL84 is affected by the deletion of IE2 40, and expression of the tegument protein pp65 (ppUL83) is affected by the deletion of both IE2 40 and IE2 60. IE2 60 and IE2 40 are also required for the production of normal levels of infectious virus. Finally, IE2 40 appears to function as a repressor of major immediate-early transcription in the infected cell. These results begin to define functions for the IE2 60- and IE2 40-kDa proteins and indicate that these products contribute both to the expression of selected viral genes and to the overall progression of the infection.
Figures





Similar articles
-
Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection.Virology. 2000 Aug 15;274(1):39-55. doi: 10.1006/viro.2000.0448. Virology. 2000. PMID: 10936087
-
Repression of HMGA2 gene expression by human cytomegalovirus involves the IE2 86-kilodalton protein and is necessary for efficient viral replication and inhibition of cyclin A transcription.J Virol. 2006 Oct;80(20):9951-61. doi: 10.1128/JVI.01300-06. J Virol. 2006. PMID: 17005673 Free PMC article.
-
Viable human cytomegalovirus recombinant virus with an internal deletion of the IE2 86 gene affects late stages of viral replication.J Virol. 2002 Mar;76(6):2973-89. doi: 10.1128/jvi.76.6.2973-2989.2002. J Virol. 2002. PMID: 11861863 Free PMC article.
-
Bright and Early: Inhibiting Human Cytomegalovirus by Targeting Major Immediate-Early Gene Expression or Protein Function.Viruses. 2020 Jan 16;12(1):110. doi: 10.3390/v12010110. Viruses. 2020. PMID: 31963209 Free PMC article. Review.
-
Activation and regulation of human cytomegalovirus early genes.Intervirology. 1996;39(5-6):361-77. doi: 10.1159/000150507. Intervirology. 1996. PMID: 9130046 Review.
Cited by
-
The human cytomegalovirus UL76 gene regulates the level of expression of the UL77 gene.PLoS One. 2010 Jul 30;5(7):e11901. doi: 10.1371/journal.pone.0011901. PLoS One. 2010. PMID: 20689582 Free PMC article.
-
Regulation of the MIE Locus During HCMV Latency and Reactivation.Pathogens. 2020 Oct 23;9(11):869. doi: 10.3390/pathogens9110869. Pathogens. 2020. PMID: 33113934 Free PMC article. Review.
-
Human cytomegalovirus and Herpes Simplex type I virus can engage RNA polymerase I for transcription of immediate early genes.Oncotarget. 2017 Oct 29;8(57):96536-96552. doi: 10.18632/oncotarget.22106. eCollection 2017 Nov 14. Oncotarget. 2017. PMID: 29228551 Free PMC article.
-
Multiple Transcripts Encode Full-Length Human Cytomegalovirus IE1 and IE2 Proteins during Lytic Infection.J Virol. 2016 Sep 12;90(19):8855-65. doi: 10.1128/JVI.00741-16. Print 2016 Oct 1. J Virol. 2016. PMID: 27466417 Free PMC article.
-
A human cytomegalovirus-encoded microRNA regulates expression of multiple viral genes involved in replication.PLoS Pathog. 2007 Nov;3(11):e163. doi: 10.1371/journal.ppat.0030163. PLoS Pathog. 2007. PMID: 17983268 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

