Multiple aromatic side chains within a disordered structure are critical for transcription and transforming activity of EWS family oncoproteins
- PMID: 17202261
- PMCID: PMC1766410
- DOI: 10.1073/pnas.0607007104
Multiple aromatic side chains within a disordered structure are critical for transcription and transforming activity of EWS family oncoproteins
Abstract
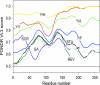
Chromosomal translocations involving the N-terminal approximately 250 residues of the Ewings sarcoma (EWS) oncogene produce a group of EWS fusion proteins (EFPs) that cause several distinct human cancers. EFPs are potent transcriptional activators and interact with other proteins required for mRNA biogenesis, indicating that EFPs induce tumorigenesis by perturbing gene expression. Although EFPs were discovered more than a decade ago, molecular analysis has been greatly hindered by the repetitive EWS activation domain (EAD) structure, containing multiple degenerate hexapeptide repeats (consensus SYGQQS) with a conserved tyrosine residue. By exploiting total gene synthesis, we have been able to systematically mutagenize the EAD and determine the effect on transcriptional activation by EWS/ATF1 and cellular transformation by EWS/Fli1. In both assays, we find the following requirements for EAD function. First, multiple tyrosine residues are essential. Second, phenylalanine can effectively substitute for tyrosine, showing that an aromatic ring can confer EAD function in the absence of tyrosine phosphorylation. Third, there is little requirement for specific peptide sequences and, thus, overall sequence composition (and not the degenerate hexapeptide repeat) confers EAD activity. Consistent with the above findings, we also report that the EAD is intrinsically disordered. However, a sensitive computational predictor of natural protein disorder (PONDR VL3) identifies potential molecular recognition features that are tyrosine-dependent and that correlate well with EAD function. In summary we have uncovered several molecular features of the EAD that will impact future studies of the broader EFP family and molecular recognition by complex intrinsically disordered proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
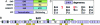



Similar articles
-
A repetitive element containing a critical tyrosine residue is required for transcriptional activation by the EWS/ATF1 oncogene.Oncogene. 2001 Jul 12;20(31):4161-8. doi: 10.1038/sj.onc.1204522. Oncogene. 2001. PMID: 11464282
-
Mapping the Structure-Function Relationships of Disordered Oncogenic Transcription Factors Using Transcriptomic Analysis.J Vis Exp. 2020 Jun 27;(160):10.3791/61564. doi: 10.3791/61564. J Vis Exp. 2020. PMID: 32658189 Free PMC article.
-
Disordered binding regions of Ewing's sarcoma fusion proteins.Bioorg Khim. 2014 Jan-Feb;40(1):20-30. Bioorg Khim. 2014. PMID: 25898720
-
Molecular recognition by the EWS transcriptional activation domain.Adv Exp Med Biol. 2012;725:106-25. doi: 10.1007/978-1-4614-0659-4_7. Adv Exp Med Biol. 2012. PMID: 22399321 Review.
-
Ewings family oncoproteins: drunk, disorderly and in search of partners.Cell Res. 2007 Apr;17(4):286-8. doi: 10.1038/cr.2007.22. Cell Res. 2007. PMID: 17426699 Review. No abstract available.
Cited by
-
Recent advances in targeted therapy for Ewing sarcoma.F1000Res. 2016 Aug 25;5:F1000 Faculty Rev-2077. doi: 10.12688/f1000research.8631.1. eCollection 2016. F1000Res. 2016. PMID: 27635231 Free PMC article. Review.
-
Single enantiomer of YK-4-279 demonstrates specificity in targeting the oncogene EWS-FLI1.Oncotarget. 2012 Feb;3(2):172-82. doi: 10.18632/oncotarget.454. Oncotarget. 2012. PMID: 22383402 Free PMC article.
-
Insights into Molecular Diversity within the FET Family: Unraveling Phase Separation of the N-Terminal Low Complexity Domain from RNA-Binding Protein EWS.bioRxiv [Preprint]. 2023 Nov 1:2023.10.27.564484. doi: 10.1101/2023.10.27.564484. bioRxiv. 2023. Update in: J Am Chem Soc. 2024 Mar 27;146(12):8071-8085. doi: 10.1021/jacs.3c12034. PMID: 37961424 Free PMC article. Updated. Preprint.
-
An overview of the importance of conformational flexibility in gene regulation by the transcription factors.J Biophys. 2009;2009:210485. doi: 10.1155/2009/210485. Epub 2010 Feb 4. J Biophys. 2009. PMID: 20169123 Free PMC article.
-
In vitro interaction between the N-terminus of the Ewing's sarcoma protein and the subunit of RNA polymerase II hsRPB7.Mol Biol Rep. 2009 Jul;36(6):1269-74. doi: 10.1007/s11033-008-9308-2. Epub 2008 Jul 8. Mol Biol Rep. 2009. PMID: 18607770
References
-
- Kim J, Pelletier J. Physiol Genomics. 1999;1:127–138. - PubMed
-
- Arvand A, Denny CT. Oncogene. 2001;20:5747–5754. - PubMed
-
- Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, Kovar H, Joubert I, de Jong P, Rouleau G, et al. Nature. 1992;359:162–165. - PubMed
-
- Uversky VN, Oldfield CJ, Dunker AK. J Mol Recognit. 2005;18:343–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

