Impact of spiramycin treatment and gestational age on maturation of Toxoplasma gondii immunoglobulin G avidity in pregnant women
- PMID: 17202303
- PMCID: PMC1828858
- DOI: 10.1128/CVI.00311-06
Impact of spiramycin treatment and gestational age on maturation of Toxoplasma gondii immunoglobulin G avidity in pregnant women
Abstract
The objective of the present study was to investigate the maturation of immunoglobulin G (IgG) avidity after Toxoplasma gondii seroconversion during pregnancy and the factors that affect IgG avidity over time. The study used 309 serum samples from 117 women and a multiple linear mixed regression analysis to show the patterns of variation of IgG avidity throughout gestation. The IgG avidity ratios and the patterns of their evolution with time were quite diverse among the women and were statistically heterogeneous (P = 0.011); however, the trend was toward a statistically significant increase (P < 0.0001). On average, a 1.0167-fold increase was observed for each additional gestational week after the putative date of infection. At 12 weeks after putative infection (the expected IgG avidity maturation time), the mean avidity ratio was 16.6% (95% confidence interval, 15.4 to 17.9%). At all times, the avidity ratio remained significantly heterogeneous among the women (P < 0.05); for 95% of them, that ratio ranged from 7.8 to 35.3% at 12 weeks after putative infection. Maternal age at the putative time of infection did not influence the maturation of IgG avidity. However, on average, a 1.009-fold decrease (P = 0.03) in that avidity was observed for each additional week of gestational age before infection and a 1.03-fold increase (P = 0.0003) was observed for each additional week of delay to the onset of spiramycin treatment. The rate of increase in the avidity ratio was lower if infection occurred late in pregnancy and higher if the delay to treatment was long. This information cannot allow accurate determination of the delay since the time of infection. The present results provide support for interpretation of the assay and caution against overinterpretation.
Figures

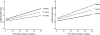
Similar articles
-
Spiramycin treatment of Toxoplasma gondii infection in pregnant women impairs the production and the avidity maturation of T. gondii-specific immunoglobulin G antibodies.Clin Vaccine Immunol. 2009 Oct;16(10):1517-20. doi: 10.1128/CVI.00253-09. Epub 2009 Aug 19. Clin Vaccine Immunol. 2009. PMID: 19692628 Free PMC article.
-
Identification, production and assessment of two Toxoplasma gondii recombinant proteins for use in a Toxoplasma IgG avidity assay.Pathog Glob Health. 2016 Oct-Dec;110(7-8):277-286. doi: 10.1080/20477724.2016.1238186. Epub 2016 Oct 4. Pathog Glob Health. 2016. PMID: 27697019 Free PMC article.
-
Comparison of Toxoplasma gondii IgG avidity Architect and Vidas assays with the estimated date of infection in pregnant women.Parasite. 2016;23:45. doi: 10.1051/parasite/2016056. Epub 2016 Oct 20. Parasite. 2016. PMID: 27762213 Free PMC article.
-
Delayed maturation of immunoglobulin G avidity: implication for the diagnosis of toxoplasmosis in pregnant women.Eur J Clin Microbiol Infect Dis. 2006 Nov;25(11):687-93. doi: 10.1007/s10096-006-0204-1. Eur J Clin Microbiol Infect Dis. 2006. PMID: 17024503 Review.
-
Toxoplasma gondii-specific IgG avidity testing in pregnant women.Clin Microbiol Infect. 2020 Sep;26(9):1155-1160. doi: 10.1016/j.cmi.2020.04.014. Epub 2020 Apr 22. Clin Microbiol Infect. 2020. PMID: 32334096 Review.
Cited by
-
Help in the Choice of Automated or Semiautomated Immunoassays for Serological Diagnosis of Toxoplasmosis: Evaluation of Nine Immunoassays by the French National Reference Center for Toxoplasmosis.J Clin Microbiol. 2016 Dec;54(12):3034-3042. doi: 10.1128/JCM.01193-16. Epub 2016 Oct 12. J Clin Microbiol. 2016. PMID: 27733631 Free PMC article.
-
Persistent Low Toxoplasma IgG Avidity Is Common in Pregnancy: Experience from Antenatal Testing in Norway.PLoS One. 2015 Dec 29;10(12):e0145519. doi: 10.1371/journal.pone.0145519. eCollection 2015. PLoS One. 2015. PMID: 26714282 Free PMC article.
-
Antiparasitic treatment suppresses production and avidity of Toxoplasma gondii-specific antibodies in a murine model of acute infection*.Eur J Microbiol Immunol (Bp). 2011 Sep;1(3):249-55. doi: 10.1556/EuJMI.1.2011.3.9. Epub 2011 Sep 9. Eur J Microbiol Immunol (Bp). 2011. PMID: 24516731 Free PMC article.
-
Epidemiology of and diagnostic strategies for toxoplasmosis.Clin Microbiol Rev. 2012 Apr;25(2):264-96. doi: 10.1128/CMR.05013-11. Clin Microbiol Rev. 2012. PMID: 22491772 Free PMC article. Review.
-
Comparison of four commercially available avidity tests for Toxoplasma gondii-specific IgG antibodies.Clin Vaccine Immunol. 2013 Feb;20(2):197-204. doi: 10.1128/CVI.00356-12. Epub 2012 Dec 12. Clin Vaccine Immunol. 2013. PMID: 23239801 Free PMC article.
References
-
- Buffolano, W., M. Lappalainen, L. Hedman, F. Ciccimarra, M. Del Pezzo, R. Rescaldani, N. Gargano, and K. Hedman. 2004. Delayed maturation of IgG avidity in congenital toxoplasmosis. Eur. J. Clin. Microbiol. Infect. Dis. 23:825-830. - PubMed
-
- Cozon, G. J., J. Ferrandiz, H. Nebhi, M. Wallon, and F. Peyron. 1998. Estimation of the avidity of immunoglobulin G for routine diagnosis of chronic Toxoplasma gondii infection in pregnant women. Eur. J. Clin. Microbiol. Infect. Dis. 17:32-36. - PubMed
-
- Derouin, F. 2001. Anti-toxoplasmosis drugs. Curr. Opin. Investig. Drugs 2:1368-1374. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

