Intranasal coadministration of live lactococci producing interleukin-12 and a major cow's milk allergen inhibits allergic reaction in mice
- PMID: 17202306
- PMCID: PMC1828845
- DOI: 10.1128/CVI.00299-06
Intranasal coadministration of live lactococci producing interleukin-12 and a major cow's milk allergen inhibits allergic reaction in mice
Abstract
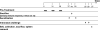
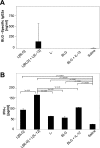
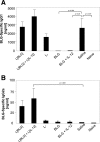
The Th1/Th2 balance deregulation toward a Th2 immune response plays a central role in allergy. We previously demonstrated that administration of recombinant Lactococcus lactis strains expressing bovine beta-lactoglobulin (BLG), a major cow's milk allergen, partially prevents mice from sensitization. In the present study, we aimed to improve this preventive effect by coadministration of L. lactis BLG and a second recombinant L. lactis strain producing biologically active interleukin-12 (IL-12). This L. lactis strain producing IL-12 was previously used to enhance the Th1 immune response in a tumoral murine model (L. G. Bermúdez-Humarán et al., J. Immunol. 175:7297-7302, 2005). A comparison of the administration of either BLG alone or BLG in the presence of IL-12 was conducted. A BLG-specific primary Th1 immune response was observed only after intranasal coadministration of both L. lactis BLG and IL-12-producing L. lactis, as demonstrated by the induction of serum-specific immunoglobulin G2a (IgG2a) concomitant with gamma interferon secretion by splenocytes, confirming the adjuvanticity of IL-12-producing L. lactis. Immunized mice were further sensitized by intraperitoneal administration of purified BLG, and the allergic reaction was elicited by intranasal challenge with purified BLG. Mice pretreated with BLG in either the presence or the absence of IL-12 were rendered completely tolerant to further allergic sensitization and elicitation. Pretreatment with either L. lactis BLG or L. lactis BLG and IL-12-producing L. lactis induces specific anti-BLG IgG2a production in serum and bronchoalveolar lavage (BAL) fluid. Although specific serum IgE was not affected by these pretreatments, the levels of eosinophilia and IL-5 secretion in BAL fluid were significantly reduced after BLG challenge in the groups pretreated with L. lactis BLG and L. lactis BLG-IL-12-producing L. lactis, demonstrating a decreased allergic reaction. Our data demonstrate for the first time (i) the induction of a protective Th1 response by the association of L. lactis BLG and IL-12-producing L. lactis which inhibits the elicitation of the allergic reaction to BLG in mice and (ii) the efficiency of intranasal administration of BLG for the induction of tolerance.
Figures


Similar articles
-
Allergy therapy by intranasal administration with recombinant Lactococcus lactis Producing bovine beta-lactoglobulin.Int Arch Allergy Immunol. 2009;150(1):25-31. doi: 10.1159/000210377. Epub 2009 Apr 2. Int Arch Allergy Immunol. 2009. PMID: 19339799
-
Oral administration of recombinant Lactococcus lactis expressing bovine beta-lactoglobulin partially prevents mice from sensitization.Clin Exp Allergy. 2005 Apr;35(4):539-46. doi: 10.1111/j.1365-2222.2005.02225.x. Clin Exp Allergy. 2005. PMID: 15836765
-
Induction of mucosal immune response after intranasal or oral inoculation of mice with Lactococcus lactis producing bovine beta-lactoglobulin.Clin Diagn Lab Immunol. 2001 May;8(3):545-51. doi: 10.1128/CDLI.8.3.545-551.2001. Clin Diagn Lab Immunol. 2001. PMID: 11329455 Free PMC article.
-
Current prophylactic and therapeutic uses of a recombinant Lactococcus lactis strain secreting biologically active interleukin-12.J Mol Microbiol Biotechnol. 2008;14(1-3):80-9. doi: 10.1159/000106086. J Mol Microbiol Biotechnol. 2008. PMID: 17957114 Review.
-
The extended farm effect: The milk protein β-lactoglobulin in stable dust protects against allergies.Allergol Select. 2022 Mar 29;6:111-117. doi: 10.5414/ALX02246E. eCollection 2022. Allergol Select. 2022. PMID: 35392214 Free PMC article. Review.
Cited by
-
Food allergy diagnosis and therapy: where are we now?Immunotherapy. 2013 Sep;5(9):931-44. doi: 10.2217/imt.13.93. Immunotherapy. 2013. PMID: 23998729 Free PMC article. Review.
-
Lactococci and lactobacilli as mucosal delivery vectors for therapeutic proteins and DNA vaccines.Microb Cell Fact. 2011 Aug 30;10 Suppl 1(Suppl 1):S4. doi: 10.1186/1475-2859-10-S1-S4. Epub 2011 Aug 30. Microb Cell Fact. 2011. PMID: 21995317 Free PMC article. Review.
-
Recombinant Invasive Lactococcus lactis Carrying a DNA Vaccine Coding the Ag85A Antigen Increases INF-γ, IL-6, and TNF-α Cytokines after Intranasal Immunization.Front Microbiol. 2017 Jul 11;8:1263. doi: 10.3389/fmicb.2017.01263. eCollection 2017. Front Microbiol. 2017. PMID: 28744263 Free PMC article.
-
Review: The Nose as a Route for Therapy. Part 2 Immunotherapy.Front Allergy. 2021 Jul 1;2:668781. doi: 10.3389/falgy.2021.668781. eCollection 2021. Front Allergy. 2021. PMID: 35387044 Free PMC article. Review.
-
Food allergy: recent advances in pathophysiology and treatment.Allergy Asthma Immunol Res. 2009 Oct;1(1):19-29. doi: 10.4168/aair.2009.1.1.19. Epub 2009 Sep 25. Allergy Asthma Immunol Res. 2009. PMID: 20224666 Free PMC article.
References
-
- Adel-Patient, K., S. Ah-Leung, C. Creminon, S. Nouaille, J. M. Chatel, P. Langella, and J. M. Wal. 2005. Oral administration of recombinant Lactococcus lactis expressing bovine beta-lactoglobulin partially prevents mice from sensitization. Clin. Exp. Allergy 35:539-546. - PubMed
-
- Adel-Patient, K., M. A. Nahori, B. Proust, J. R. Lapa e Silva, C. Creminon, J. M. Wal, and B. B. Vargaftig. 2003. Elicitation of the allergic reaction in beta-lactoglobulin-sensitized Balb/c mice: biochemical and clinical manifestations differ according to the structure of the allergen used for challenge. Clin. Exp. Allergy 33:376-385. - PubMed
-
- Adel-Patient, K., C. Creminon, D. Boquet, J. M. Wal, and J. M. Chatel. 2001. Genetic immunisation with bovine beta-lactoglobulin cDNA induces a preventive and persistent inhibition of specific anti-BLG IgE response in mice. Int. Arch. Allergy Immunol. 126:59-67. - PubMed
-
- Adel-Patient, K., C. Creminon, H. Bernard, G. Clement, L. Negroni, Y. Frobert, J. Grassi, J. M. Wal, and J. M. Chatel. 2000. Evaluation of a high IgE-responder mouse model of allergy to bovine beta-lactoglobulin (BLG): development of sandwich immunoassays for total and allergen-specific IgE, IgG1 and IgG2a in BLG-sensitized mice. J. Immunol. Methods 235:21-32. - PubMed
-
- Alvarez, D., F. K. Swirski, T. C. Yang, R. Fattouh, K. Croitoru, J. L. Bramson, M. R. Stämpfli, and M. Jordana. 2006. Inhalation tolerance is induced selectively in thoracic lymph nodes but executed pervasively at distant mucosal and non mucosal tissues. J. Immunol. 176:2568-2580. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

