Opposing actions of chronic Delta9-tetrahydrocannabinol and cannabinoid antagonists on hippocampal long-term potentiation
- PMID: 17202425
- PMCID: PMC1828281
- DOI: 10.1101/lm.439007
Opposing actions of chronic Delta9-tetrahydrocannabinol and cannabinoid antagonists on hippocampal long-term potentiation
Abstract
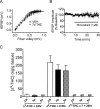
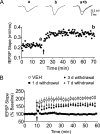
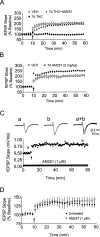
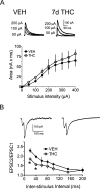
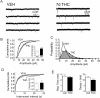
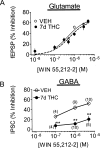
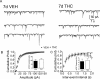
Memory deficits produced by marijuana arise partly via interaction of the psychoactive component, Delta(9)-tetrahydrocannabinol (Delta(9)-THC), with cannabinoid receptors in the hippocampus. Although cannabinoids acutely reduce glutamate release and block hippocampal long-term potentiation (LTP), a potential substrate for learning and memory, the consequences of prolonged exposure to Delta(9)-THC for hippocampal function are poorly understood. Rats were injected with Delta(9)-THC (10 mg/kg, i.p., q.d.) for 1, 3, or 7 d, and electrophysiological recordings were performed in hippocampal slices 1d after the final injection. At this time, Delta(9)-THC was undetectable in hippocampus using liquid chromatography-mass spectrometry (LC-MS). Hippocampal LTP generated using high-frequency (HFS) or theta burst stimulation was not observed in brain slices from the 7-d Delta(9)-THC-treated animals. Delta(9)-THC also blocked HFS-LTP after 3 d, but not 1 d of treatment. The complete blockade of LTP persisted for 3 d after the last Delta(9)-THC injection, and full reversal of the LTP deficit was not observed up to 14 d following Delta(9)-THC withdrawal. The cannabinoid antagonist AM251 (2 mg/kg), administered before each Delta(9)-THC injection prevented the blockade of LTP, and 7-d treatment with AM251 alone significantly increased the level of LTP. Chronic Delta(9)-THC also produced tolerance to the inhibition of synaptic GABA, but not glutamate release by the agonist WIN55,212-2. These data define consequences of repeated Delta(9)-THC exposure for synaptic plasticity in the hippocampus that may help explain memory impairments in humans following chronic marijuana use.
Figures

Similar articles
-
Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure.J Neurosci. 2003 Jun 15;23(12):4815-20. doi: 10.1523/JNEUROSCI.23-12-04815.2003. J Neurosci. 2003. PMID: 12832502 Free PMC article.
-
Disruption of hippocampal synaptic transmission and long-term potentiation by psychoactive synthetic cannabinoid 'Spice' compounds: comparison with Δ9 -tetrahydrocannabinol.Addict Biol. 2017 Mar;22(2):390-399. doi: 10.1111/adb.12334. Epub 2016 Jan 5. Addict Biol. 2017. PMID: 26732435 Free PMC article.
-
Differential effects of the sleep-inducing lipid oleamide and cannabinoids on the induction of long-term potentiation in the CA1 neurons of the rat hippocampus in vitro.Brain Res. 2004 Jan 30;997(1):1-14. doi: 10.1016/j.brainres.2003.10.019. Brain Res. 2004. PMID: 14715144
-
Cannabinoids, hippocampal function and memory.Life Sci. 1999;65(6-7):715-23. doi: 10.1016/s0024-3205(99)00294-5. Life Sci. 1999. PMID: 10462072 Review.
-
Pharmacological and Toxicological Effects of Synthetic Cannabinoids and Their Metabolites.Curr Top Behav Neurosci. 2017;32:249-262. doi: 10.1007/7854_2016_60. Curr Top Behav Neurosci. 2017. PMID: 28012093 Free PMC article. Review.
Cited by
-
Cannabis, Cigarettes, and Their Co-Occurring Use: Disentangling Differences in Gray Matter Volume.Int J Neuropsychopharmacol. 2015 Jun 4;18(10):pyv061. doi: 10.1093/ijnp/pyv061. Int J Neuropsychopharmacol. 2015. PMID: 26045474 Free PMC article.
-
Nigrostriatal denervation changes the effect of cannabinoids on subthalamic neuronal activity in rats.Psychopharmacology (Berl). 2011 Mar;214(2):379-89. doi: 10.1007/s00213-010-2043-0. Epub 2010 Oct 20. Psychopharmacology (Berl). 2011. PMID: 20959968 Free PMC article.
-
Cell-specific STORM super-resolution imaging reveals nanoscale organization of cannabinoid signaling.Nat Neurosci. 2015 Jan;18(1):75-86. doi: 10.1038/nn.3892. Epub 2014 Dec 8. Nat Neurosci. 2015. PMID: 25485758 Free PMC article.
-
Cannabinoids, Endocannabinoids and Sleep.Front Mol Neurosci. 2020 Jul 22;13:125. doi: 10.3389/fnmol.2020.00125. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32774241 Free PMC article.
-
Synaptic targets of Δ9-tetrahydrocannabinol in the central nervous system.Cold Spring Harb Perspect Med. 2013 Aug 1;3(8):a012237. doi: 10.1101/cshperspect.a012237. Cold Spring Harb Perspect Med. 2013. PMID: 23209160 Free PMC article. Review.
References
-
- Bliss T.V., Collingridge G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
-
- Blitzer R.D., Wong T., Nouranifar R., Iyengar R., Landau E.M. Postsynaptic CAMP pathway gates early LTP in hippocampal CA1 region. Neuron. 1995;15:1403–1414. - PubMed
-
- Bohme G.A., Laville M., Ledent C., Parmentier M., Imperato A. Enhanced long-term potentiation in mice lacking cannabinoid CB1 receptors. Neuroscience. 2000;95:5–7. - PubMed
-
- Bolla K.I., Brown K., Eldreth D., Tate K., Cadet J.L. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. - PubMed
-
- Boyd S.T., Fremming B.A. Rimonabant—A selective CB1 antagonist. Ann. Pharmacother. 2005;39:684–690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
