Associative representational plasticity in the auditory cortex: a synthesis of two disciplines
- PMID: 17202426
- PMCID: PMC3601844
- DOI: 10.1101/lm.421807
Associative representational plasticity in the auditory cortex: a synthesis of two disciplines
Abstract
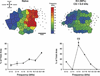
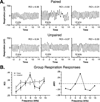
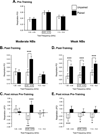
Historically, sensory systems have been largely ignored as potential loci of information storage in the neurobiology of learning and memory. They continued to be relegated to the role of "sensory analyzers" despite consistent findings of associatively induced enhancement of responses in primary sensory cortices to behaviorally important signal stimuli, such as conditioned stimuli (CS), during classical conditioning. This disregard may have been promoted by the fact that the brain was interrogated using only one or two stimuli, e.g., a CS(+) sometimes with a CS(-), providing little insight into the specificity of neural plasticity. This review describes a novel approach that synthesizes the basic experimental designs of the experimental psychology of learning with that of sensory neurophysiology. By probing the brain with a large stimulus set before and after learning, this unified method has revealed that associative processes produce highly specific changes in the receptive fields of cells in the primary auditory cortex (A1). This associative representational plasticity (ARP) selectively facilitates responses to tonal CSs at the expense of other frequencies, producing tuning shifts toward and to the CS and expanded representation of CS frequencies in the tonotopic map of A1. ARPs have the major characteristics of associative memory: They are highly specific, discriminative, rapidly acquired, exhibit consolidation over hours and days, and can be retained indefinitely. Evidence to date suggests that ARPs encode the level of acquired behavioral importance of stimuli. The nucleus basalis cholinergic system is sufficient both for the induction of ARPs and the induction of specific auditory memory. Investigation of ARPs has attracted workers with diverse backgrounds, often resulting in behavioral approaches that yield data that are difficult to interpret. The advantages of studying associative representational plasticity are emphasized, as is the need for greater behavioral sophistication.
Figures




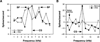



Comment in
-
The neural circuit for tone-specific plasticity in the auditory system elicited by conditioning.Learn Mem. 2008 Mar 20;15(4):198-201; author reply 202-7. doi: 10.1101/lm.791408. Print 2008 Apr. Learn Mem. 2008. PMID: 18385473 No abstract available.
Similar articles
-
Auditory associative memory and representational plasticity in the primary auditory cortex.Hear Res. 2007 Jul;229(1-2):54-68. doi: 10.1016/j.heares.2007.01.004. Epub 2007 Jan 17. Hear Res. 2007. PMID: 17344002 Free PMC article. Review.
-
Physiological memory in primary auditory cortex: characteristics and mechanisms.Neurobiol Learn Mem. 1998 Jul-Sep;70(1-2):226-51. doi: 10.1006/nlme.1998.3850. Neurobiol Learn Mem. 1998. PMID: 9753599 Review.
-
Learning-induced physiological memory in adult primary auditory cortex: receptive fields plasticity, model, and mechanisms.Audiol Neurootol. 1998 Mar-Jun;3(2-3):145-67. doi: 10.1159/000013787. Audiol Neurootol. 1998. PMID: 9575382 Review.
-
The nucleus basalis and memory codes: auditory cortical plasticity and the induction of specific, associative behavioral memory.Neurobiol Learn Mem. 2003 Nov;80(3):268-84. doi: 10.1016/s1074-7427(03)00072-8. Neurobiol Learn Mem. 2003. PMID: 14521869 Review.
-
New perspectives on the auditory cortex: learning and memory.Handb Clin Neurol. 2015;129:117-47. doi: 10.1016/B978-0-444-62630-1.00007-X. Handb Clin Neurol. 2015. PMID: 25726266 Review.
Cited by
-
Perceiving threat in the face of safety: excitation and inhibition of conditioned fear in human visual cortex.J Neurosci. 2013 Jan 2;33(1):72-8. doi: 10.1523/JNEUROSCI.3692-12.2013. J Neurosci. 2013. PMID: 23283323 Free PMC article.
-
Toward an account of clinical anxiety predicated on basic, neurally mapped mechanisms of Pavlovian fear-learning: the case for conditioned overgeneralization.Depress Anxiety. 2012 Apr;29(4):257-63. doi: 10.1002/da.21922. Epub 2012 Mar 23. Depress Anxiety. 2012. PMID: 22447565 Free PMC article. Review.
-
Detection of an inhibitory cortical gradient underlying peak shift in learning: a neural basis for a false memory.Neurobiol Learn Mem. 2012 Nov;98(4):368-79. doi: 10.1016/j.nlm.2012.10.001. Epub 2012 Oct 11. Neurobiol Learn Mem. 2012. PMID: 23063933 Free PMC article.
-
The hippocampus, medial prefrontal cortex, and selective memory retrieval: evidence from a rodent model of the retrieval-induced forgetting effect.Hippocampus. 2014 Sep;24(9):1070-80. doi: 10.1002/hipo.22291. Epub 2014 Apr 29. Hippocampus. 2014. PMID: 24753146 Free PMC article.
-
Histone Deacetylase Inhibition via RGFP966 Releases the Brakes on Sensory Cortical Plasticity and the Specificity of Memory Formation.J Neurosci. 2015 Sep 23;35(38):13124-32. doi: 10.1523/JNEUROSCI.0914-15.2015. J Neurosci. 2015. PMID: 26400942 Free PMC article.
References
-
- Aramakis VB, Bandrowski AE, Ashe JH. Muscarinic reduction of GABAergic synaptic potentials results in disinhibition of the AMPA/kainate-mediated EPSP in auditory cortex. Brain Res. 1997;758:107–117. - PubMed
-
- Aramakis VB, Bandrowski AE, Ashe JH. Role of muscarinic receptors, G-proteins, and intracellular messengers in muscarinic modulation of NMDA receptor-mediated synaptic transmission. Synapse. 1999;32:262–275. - PubMed
-
- Ashe JH, McKenna TM, Weinberger NM. Cholinergic modulation of frequency receptive fields in auditory cortex: II. Frequency-specific effects of anticholinesterases provide evidence for a modulatory action of endogenous ACh. Synapse. 1989;4:44–54. - PubMed
-
- Bakin JS, Weinberger NM. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 1990;536:271–286. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
