Passive immunization with anti-ganglioside antibodies directly inhibits axon regeneration in an animal model
- PMID: 17202469
- PMCID: PMC6672271
- DOI: 10.1523/JNEUROSCI.4017-06.2007
Passive immunization with anti-ganglioside antibodies directly inhibits axon regeneration in an animal model
Abstract
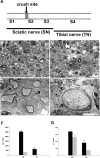
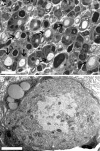
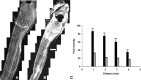
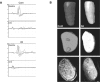
Recent studies have proposed that neurite outgrowth is influenced by specific nerve cell surface gangliosides, which are sialic acid-containing glycosphingolipids highly enriched in the mammalian nervous system. For example, the endogenous lectin, myelin-associated glycoprotein (MAG), is reported to bind to axonal gangliosides (GD1a and GT1b) to inhibit neurite outgrowth. Clustering of gangliosides in the absence of inhibitors such as MAG is also shown to inhibit neurite outgrowth in culture. In some human autoimmune PNS and CNS disorders, autoantibodies against GD1a or other gangliosides are implicated in pathophysiology. Because of neurobiological and clinical relevance, we asked whether anti-GD1a antibodies inhibit regeneration of injured axons in vivo. Passive transfer of anti-GD1a antibody severely inhibited axon regeneration after PNS injury in mice. In mutant mice with altered ganglioside or complement expression, inhibition by antibodies was mediated directly through GD1a and was independent of complement-induced cytolytic injury. The impaired regenerative responses and ultrastructure of injured peripheral axons mimicked the abortive regeneration typically seen after CNS injury. These data demonstrate that inhibition of axon regeneration is induced directly by engaging cell surface gangliosides in vivo and imply that circulating autoimmune antibodies can inhibit axon regeneration through neuronal gangliosides independent of endogenous regeneration inhibitors such as MAG.
Figures

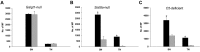
Similar articles
-
Tumor necrosis factor α receptor 1A transduces the inhibitory effect on axon regeneration triggered by IgG anti-ganglioside GD1a antibodies.Biochim Biophys Acta Mol Basis Dis. 2024 Oct;1870(7):167315. doi: 10.1016/j.bbadis.2024.167315. Epub 2024 Jun 17. Biochim Biophys Acta Mol Basis Dis. 2024. PMID: 38897255
-
Gangliosides are functional nerve cell ligands for myelin-associated glycoprotein (MAG), an inhibitor of nerve regeneration.Proc Natl Acad Sci U S A. 2002 Jun 11;99(12):8412-7. doi: 10.1073/pnas.072211699. Proc Natl Acad Sci U S A. 2002. PMID: 12060784 Free PMC article.
-
Myelin-associated glycoprotein and complementary axonal ligands, gangliosides, mediate axon stability in the CNS and PNS: neuropathology and behavioral deficits in single- and double-null mice.Exp Neurol. 2005 Sep;195(1):208-17. doi: 10.1016/j.expneurol.2005.04.017. Exp Neurol. 2005. PMID: 15953602 Free PMC article.
-
Brain gangliosides in axon-myelin stability and axon regeneration.FEBS Lett. 2010 May 3;584(9):1741-7. doi: 10.1016/j.febslet.2009.10.011. Epub 2009 Oct 12. FEBS Lett. 2010. PMID: 19822144 Free PMC article. Review.
-
Brain gangliosides: functional ligands for myelin stability and the control of nerve regeneration.Biochimie. 2001 Jul;83(7):677-82. doi: 10.1016/s0300-9084(01)01308-6. Biochimie. 2001. PMID: 11522397 Review.
Cited by
-
Anti-Chol-1 antigen, GQ1bα, antibodies are associated with Alzheimer's disease.PLoS One. 2013 May 23;8(5):e63326. doi: 10.1371/journal.pone.0063326. Print 2013. PLoS One. 2013. PMID: 23717411 Free PMC article.
-
Fcγ receptor-mediated inflammation inhibits axon regeneration.PLoS One. 2014 Feb 11;9(2):e88703. doi: 10.1371/journal.pone.0088703. eCollection 2014. PLoS One. 2014. PMID: 24523933 Free PMC article.
-
Fluorescently-tagged anti-ganglioside antibody selectively identifies peripheral nerve in living animals.Sci Rep. 2015 Oct 30;5:15766. doi: 10.1038/srep15766. Sci Rep. 2015. PMID: 26514366 Free PMC article.
-
Differential regulation of tissue-resident and blood-derived macrophages in models of autoimmune and traumatic peripheral nerve injury.Front Immunol. 2024 Nov 19;15:1487788. doi: 10.3389/fimmu.2024.1487788. eCollection 2024. Front Immunol. 2024. PMID: 39628475 Free PMC article.
-
Guillain-Barré syndrome.Nat Rev Dis Primers. 2024 Dec 19;10(1):97. doi: 10.1038/s41572-024-00580-4. Nat Rev Dis Primers. 2024. PMID: 39702645
References
-
- Acarin N, Rio J, Fernandez AL, Tintore M, Duran I, Galan I, Montalban X. Different antiganglioside antibody pattern between relapsing-remitting and progressive multiple sclerosis. Acta Neurol Scand. 1996;93:99–103. - PubMed
-
- Albers JW, Donofrio PD, McGonagle TK. Sequential electrodiagnostic abnormalities in acute inflammatory demyelinating polyradiculoneuropathy. Muscle Nerve. 1985;8:528–539. - PubMed
-
- Black MM, Lasek RJ. Slowing of the rate of axonal regeneration during growth and maturation. Exp Neurol. 1979;63:108. - PubMed
-
- Brown WF, Feasby TE. Conduction block and denervation in Guillain-Barré polyneuropathy. Brain. 1984;107:219–239. - PubMed
-
- Carpo M, Pedotti R, Allaria S, Lolli F, Mata S, Cavaletti G, Protti A, Pomati S, Scarlato G, Nobile-Orazio E. Clinical presentation and outcome of Guillain-Barré and related syndromes in relation to anti-ganglioside antibodies. J Neurol Sci. 1999;168:78–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
