Prevention of plasticity of endocannabinoid signaling inhibits persistent limbic hyperexcitability caused by developmental seizures
- PMID: 17202471
- PMCID: PMC6672287
- DOI: 10.1523/JNEUROSCI.3966-06.2007
Prevention of plasticity of endocannabinoid signaling inhibits persistent limbic hyperexcitability caused by developmental seizures
Abstract
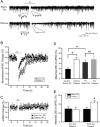
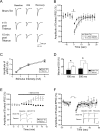
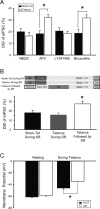
Depolarization-induced suppression of inhibition (DSI) is an endocannabinoid-mediated short-term plasticity mechanism that couples postsynaptic Ca2+ rises to decreased presynaptic GABA release. Whether the gain of this retrograde synaptic mechanism is subject to long-term modulation by glutamatergic excitatory inputs is not known. Here, we demonstrate that activity-dependent long-term DSI potentiation takes place in hippocampal slices after tetanic stimulation of Schaffer collateral synapses. This activity-dependent, long-term plasticity of endocannabinoid signaling was specific to GABAergic synapses, as it occurred without increases in the depolarization-induced suppression of excitation. Induction of tetanus-induced DSI potentiation in vitro required a complex pathway involving AMPA/kainate and metabotropic glutamate receptor as well as CB1 receptor activation. Because DSI potentiation has been suggested to play a role in persistent limbic hyperexcitability after prolonged seizures in the developing brain, we used these mechanistic insights into activity-dependent DSI potentiation to test whether interference with the induction of DSI potentiation prevents seizure-induced long-term hyperexcitability. The results showed that the in vitro, tetanus-induced DSI potentiation was occluded by previous in vivo fever-induced (febrile) seizures, indicating a common pathway. Accordingly, application of CB1 receptor antagonists during febrile seizures in vivo blocked the seizure-induced persistent DSI potentiation, abolished the seizure-induced upregulation of CB1 receptors, and prevented the emergence of long-term limbic hyperexcitability. These results reveal a new form of activity-dependent, long-term plasticity of endocannabinoid signaling at perisomatic GABAergic synapses, and demonstrate that blocking the induction of this plasticity abolishes the long-term effects of prolonged febrile seizures in the developing brain.
Figures



Similar articles
-
Long-term plasticity of endocannabinoid signaling induced by developmental febrile seizures.Neuron. 2003 Aug 14;39(4):599-611. doi: 10.1016/s0896-6273(03)00499-9. Neuron. 2003. PMID: 12925275
-
Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE).Br J Pharmacol. 2004 May;142(1):9-19. doi: 10.1038/sj.bjp.0705726. Epub 2004 Apr 20. Br J Pharmacol. 2004. PMID: 15100161 Free PMC article. Review.
-
Retrograde endocannabinoid regulation of GABAergic inhibition in the rat dentate gyrus granule cell.J Physiol. 2005 Sep 15;567(Pt 3):1001-10. doi: 10.1113/jphysiol.2005.094219. Epub 2005 Jul 21. J Physiol. 2005. PMID: 16037085 Free PMC article.
-
Characterization of the anoxia-induced long-term synaptic potentiation in area CA1 of the rat hippocampus.Br J Pharmacol. 1997 Oct;122(4):671-81. doi: 10.1038/sj.bjp.0701409. Br J Pharmacol. 1997. PMID: 9375963 Free PMC article.
-
Endocannabinoid signaling and synaptic plasticity in the brain.Crit Rev Neurobiol. 2006;18(1-2):113-24. doi: 10.1615/critrevneurobiol.v18.i1-2.120. Crit Rev Neurobiol. 2006. PMID: 17725514 Review.
Cited by
-
Cannabinoids and Epilepsy.Neurotherapeutics. 2015 Oct;12(4):747-68. doi: 10.1007/s13311-015-0375-5. Neurotherapeutics. 2015. PMID: 26282273 Free PMC article. Review.
-
2-AG-Mediated Control of GABAergic Signaling Is Impaired in a Model of Epilepsy.J Neurosci. 2023 Jan 25;43(4):571-583. doi: 10.1523/JNEUROSCI.0541-22.2022. Epub 2022 Dec 2. J Neurosci. 2023. PMID: 36460464 Free PMC article.
-
Past and present definitions of epileptogenesis and its biomarkers.Neurotherapeutics. 2014 Apr;11(2):231-41. doi: 10.1007/s13311-014-0257-2. Neurotherapeutics. 2014. PMID: 24492975 Free PMC article. Review.
-
Cholecystokinin: a multi-functional molecular switch of neuronal circuits.Dev Neurobiol. 2011 Jan 1;71(1):83-91. doi: 10.1002/dneu.20815. Dev Neurobiol. 2011. PMID: 21154912 Free PMC article. Review.
-
Endocannabinoids via CB₁ receptors act as neurogenic niche cues during cortical development.Philos Trans R Soc Lond B Biol Sci. 2012 Dec 5;367(1607):3229-41. doi: 10.1098/rstb.2011.0385. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 23108542 Free PMC article. Review.
References
-
- Annegers JF, Hauser WA, Shirts SB, Kurland LT. Factors prognostic of unprovoked seizures after febrile convulsions. N Engl J Med. 1987;316:493–498. - PubMed
-
- Beau FE, Alger BE. Transient suppression of GABAA-receptor-mediated IPSPs after epileptiform burst discharges in CA1 pyramidal cells. J Neurophysiol. 1998;79:659–669. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
