Appropriate inhibition of orexigenic hypothalamic arcuate nucleus neurons independently of leptin receptor/STAT3 signaling
- PMID: 17202473
- PMCID: PMC6672286
- DOI: 10.1523/JNEUROSCI.3168-06.2007
Appropriate inhibition of orexigenic hypothalamic arcuate nucleus neurons independently of leptin receptor/STAT3 signaling
Abstract
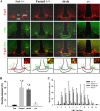
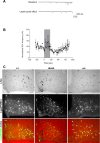
Leptin directly suppresses the activity of orexigenic neurons in the hypothalamic arcuate nucleus (ARC). We examined c-Fos-like immunoreactivity (CFLIR) as a marker of ARC neuronal activity in db/db mice devoid of the signaling form of the leptin receptor (LRb) and s/s mice that express LRb(S1138) [which is defective for STAT3 (signal transducer and activator of transcription) signaling]. Both db/db and s/s animals are hyperphagic and obese. This analysis revealed that CFLIR in agouti related peptide-expressing orexigenic ARC neurons is basally elevated in db/db but not s/s mice. Consistent with these observations, electrophysiologic evaluation of a small number of neurons in s/s animals suggested that leptin appropriately suppresses the frequency of IPSCs on ARC proopiomelanocortin (POMC) neurons that are mediated by the release of GABA from orexigenic ARC neurons. CFLIR in POMC neurons of s/s mice was also increased compared with db/db animals. Thus, these data suggest that, although LRb-->STAT3 signaling is crucial for the regulation of feeding, it is not required for the acute or chronic regulation of orexigenic ARC neurons, and the activation of STAT3-mediated transcription by leptin is not required for the appropriate development of leptin responsiveness in these neurons.
Figures

Similar articles
-
Hypothyroidism Induces Hypophagia Associated with Alterations in Protein Expression of Neuropeptide Y and Proopiomelanocortin in the Arcuate Nucleus, Independently of Hypothalamic Nuclei-Specific Changes in Leptin Signaling.Thyroid. 2016 Jan;26(1):134-43. doi: 10.1089/thy.2015.0384. Epub 2015 Dec 1. Thyroid. 2016. PMID: 26538454
-
Somato-dendritic localization and signaling by leptin receptors in hypothalamic POMC and AgRP neurons.PLoS One. 2013 Oct 29;8(10):e77622. doi: 10.1371/journal.pone.0077622. eCollection 2013. PLoS One. 2013. PMID: 24204898 Free PMC article.
-
Differential accessibility of circulating leptin to individual hypothalamic sites.Endocrinology. 2007 Nov;148(11):5414-23. doi: 10.1210/en.2007-0655. Epub 2007 Aug 9. Endocrinology. 2007. PMID: 17690165
-
LRb signals act within a distributed network of leptin-responsive neurones to mediate leptin action.Acta Physiol (Oxf). 2008 Jan;192(1):49-59. doi: 10.1111/j.1748-1716.2007.01784.x. Acta Physiol (Oxf). 2008. PMID: 18171429 Review.
-
Minireview: A hypothalamic role in energy balance with special emphasis on leptin.Endocrinology. 2004 Jun;145(6):2613-20. doi: 10.1210/en.2004-0032. Epub 2004 Mar 24. Endocrinology. 2004. PMID: 15044360 Review.
Cited by
-
Synaptic plasticity in neuronal circuits regulating energy balance.Nat Neurosci. 2012 Oct;15(10):1336-42. doi: 10.1038/nn.3219. Epub 2012 Sep 25. Nat Neurosci. 2012. PMID: 23007188 Review.
-
Enhanced hypothalamic leptin signaling in mice lacking dopamine D2 receptors.J Biol Chem. 2010 Mar 19;285(12):8905-17. doi: 10.1074/jbc.M109.079590. Epub 2010 Jan 15. J Biol Chem. 2010. PMID: 20080963 Free PMC article.
-
Modulation of Feeding and Associated Behaviors by Lateral Hypothalamic Circuits.Endocrinology. 2018 Nov 1;159(11):3631-3642. doi: 10.1210/en.2018-00449. Endocrinology. 2018. PMID: 30215694 Free PMC article. Review.
-
Leptin down-regulates KCC2 activity and controls chloride homeostasis in the neonatal rat hippocampus.Mol Brain. 2020 Nov 12;13(1):151. doi: 10.1186/s13041-020-00689-z. Mol Brain. 2020. PMID: 33183317 Free PMC article.
-
Neuroendocrine proopiomelanocortin neurons are excited by hypocretin/orexin.J Neurosci. 2009 Feb 4;29(5):1503-13. doi: 10.1523/JNEUROSCI.5147-08.2009. J Neurosci. 2009. PMID: 19193897 Free PMC article.
References
-
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. - PubMed
-
- Banks AS, Davis SM, Bates SH, Myers MG., Jr Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–14572. - PubMed
-
- Baskin DG, Schwartz MW, Seeley RJ, Woods SC, Porte D, Jr, Breininger JF, Jonak Z, Schaefer J, Krouse M, Burghardt C, Campfield LA, Burn P, Kochan JP. Leptin receptor long-form splice-variant protein expression in neuron cell bodies of the brain and co-localization with neuropeptide Y mRNA in the arcuate nucleus. J Histochem Cytochem. 1999;47:353–362. - PubMed
-
- Bates SH, Stearns WH, Schubert M, Tso AWK, Wang Y, Banks AS, Dundon TA, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signaling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. - PubMed
-
- Bates SH, Dundon TA, Seifert M, Carlson M, Maratos-Flier E, Myers MG., Jr LRb-STAT3 signaling is required for the neuroendocrine regulation of energy expenditure by leptin. Diabetes. 2004;53:3067–3073. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 DK056116/DK/NIDDK NIH HHS/United States
- R01 DK056731/DK/NIDDK NIH HHS/United States
- DK56731/DK/NIDDK NIH HHS/United States
- R01 DK062202/DK/NIDDK NIH HHS/United States
- P51 RR000163/RR/NCRR NIH HHS/United States
- DK53301/DK/NIDDK NIH HHS/United States
- DK56116/DK/NIDDK NIH HHS/United States
- R01 DK057768/DK/NIDDK NIH HHS/United States
- R37 DK056731/DK/NIDDK NIH HHS/United States
- RR0163/RR/NCRR NIH HHS/United States
- R01 DK053301/DK/NIDDK NIH HHS/United States
- DK57768/DK/NIDDK NIH HHS/United States
- R37 DK053301/DK/NIDDK NIH HHS/United States
- R56 DK062202/DK/NIDDK NIH HHS/United States
- DK62202/DK/NIDDK NIH HHS/United States
- K01 RR000163/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
